Method for preparing placenta hematopoietic stem cell preparation
A technology of hematopoietic stem cells and preparations, applied in the field of biomedicine, can solve the problems of low cell separation efficiency and inability to use directly, and achieve the effect of improving separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
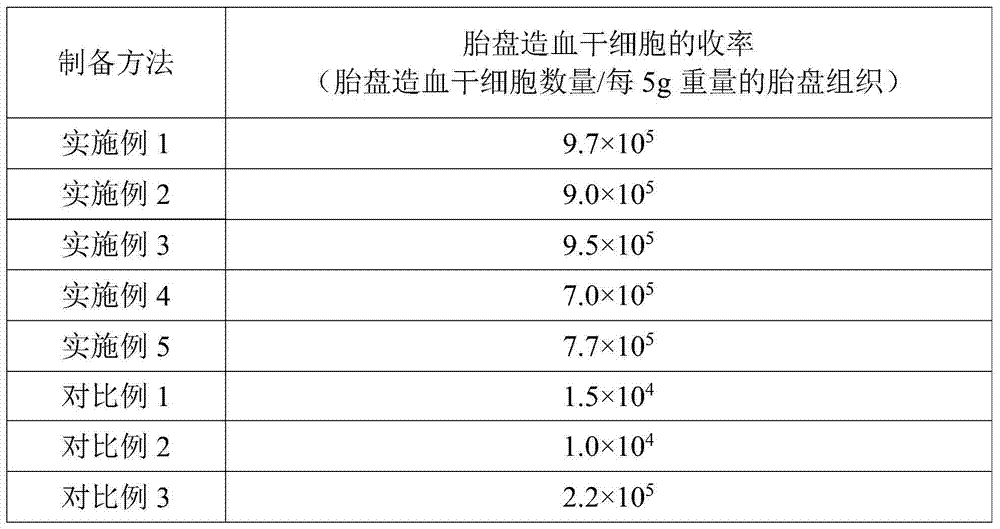
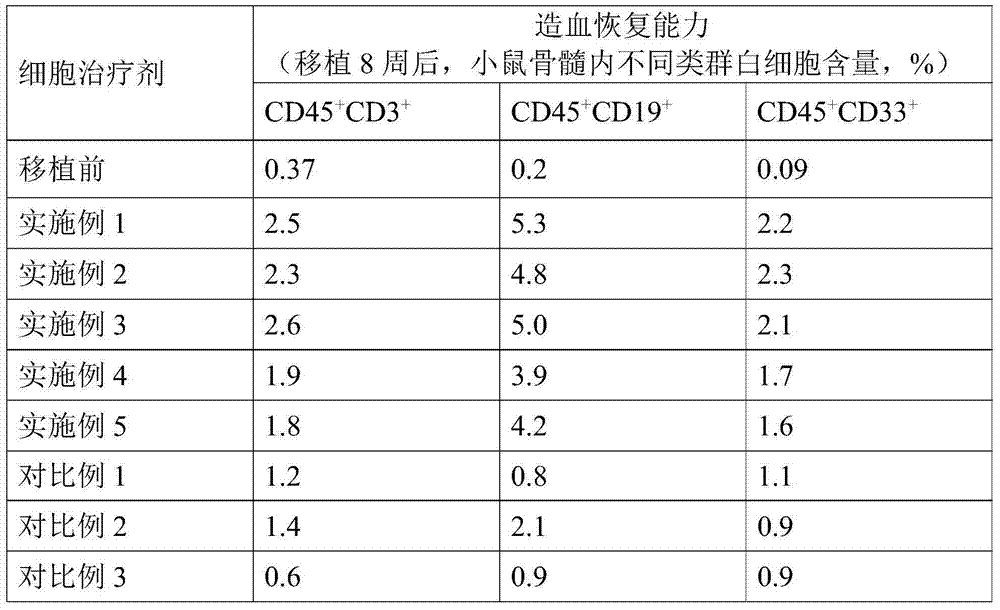
[0015] According to the present invention, wherein, according to a particularly preferred embodiment of the present invention, the enzyme solution contains 200-300 IU / mL type I collagenase, 30-50 IU / mL DNase I, 1.5-3.5 IU / mL dispase and 600-800IU / mL hyaluronidase; the freezing liquid contains 35-50mL / L dimethyl sulfoxide, 10-20g / L hydroxyethyl starch, 50-100g / L Human serum albumin, 10-20g / L dextran, 5-5.5g / L sodium chloride, 4.5-5g / L sodium gluconate, 3.4-3.9g / L sodium acetate, 0.3-0.45g / L Potassium chloride and 0.2-0.4g / L magnesium chloride. In this preferred embodiment, the present invention can further improve the separation efficiency of placental cells, and improve the hematopoietic colonization ability of frozen placental hematopoietic stem cells after returning to normal temperature.
[0016] According to the present invention, wherein, the weight ratio of the placental tissue and the enzyme solution can be 1: (0.5-5), preferably 1: (1-4); The volume ratio of the solu...
Embodiment 1
[0028] Digest the placental tissue with an enzyme solution at a temperature of 37°C for 30 minutes to obtain a digested product and filter through double-layer gauze to remove tissue residues in the digested product to obtain an enzymatic hydrolyzate; mix the enzymatically hydrolyzed solution with hydroxyethyl starch solution And let it stand for 20 minutes to separate the mixed materials to obtain the upper nucleated cell suspension; mix the nucleated cell suspension with the cryopreservation solution to obtain the placental hematopoietic stem cell preparation of this embodiment.
[0029] Wherein, the enzyme solution contains 250IU / mL type I collagenase, 40IU / mL DNase I, 2.4IU / mL dispase and 700IU / mL hyaluronidase; L of dimethyl sulfoxide, 15g / L of hydroxyethyl starch, 80g / L of human serum albumin, 15g / L of dextran, 5.2g / L of sodium chloride, 4.8g / L of sodium gluconate, 3.7g / L of sodium acetate, 0.40g / L of potassium chloride and 0.3g / L of magnesium chloride. The weight ratio...
Embodiment 2
[0031] Digest the placental tissue with an enzyme solution at a temperature of 37°C for 30 minutes to obtain a digested product and filter through double-layer gauze to remove tissue residues in the digested product to obtain an enzymatic hydrolyzate; mix the enzymatically hydrolyzed solution with hydroxyethyl starch solution And let it stand for 20 minutes to separate the mixed materials to obtain the upper nucleated cell suspension; mix the nucleated cell suspension with the cryopreservation solution to obtain the placental hematopoietic stem cell preparation of this embodiment.
[0032] Wherein, the enzyme solution contains 200IU / mL type I collagenase, 50IU / mL DNase I, 1.5IU / mL dispase and 800IU / mL hyaluronidase; L of dimethyl sulfoxide, 10g / L of hydroxyethyl starch, 100g / L of human serum albumin, 20g / L of dextran, 5g / L of sodium chloride, 5.0g / L of sodium gluconate, 3.4 g / L sodium acetate, 0.30g / L potassium chloride and 0.4g / L magnesium chloride. The weight ratio of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com