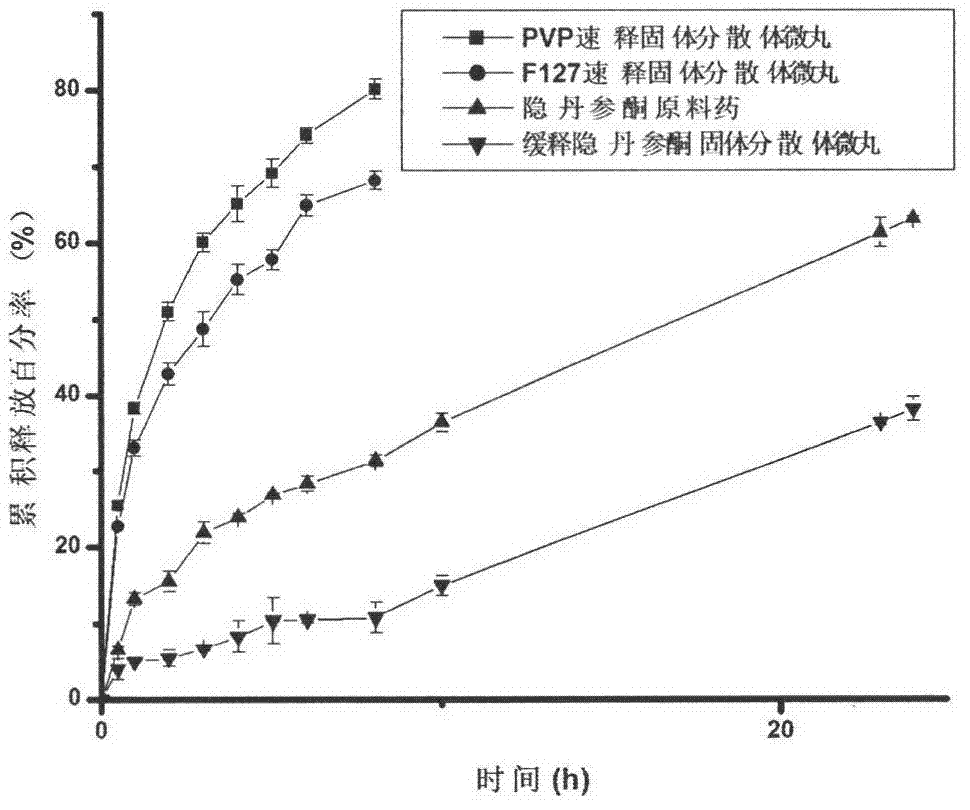
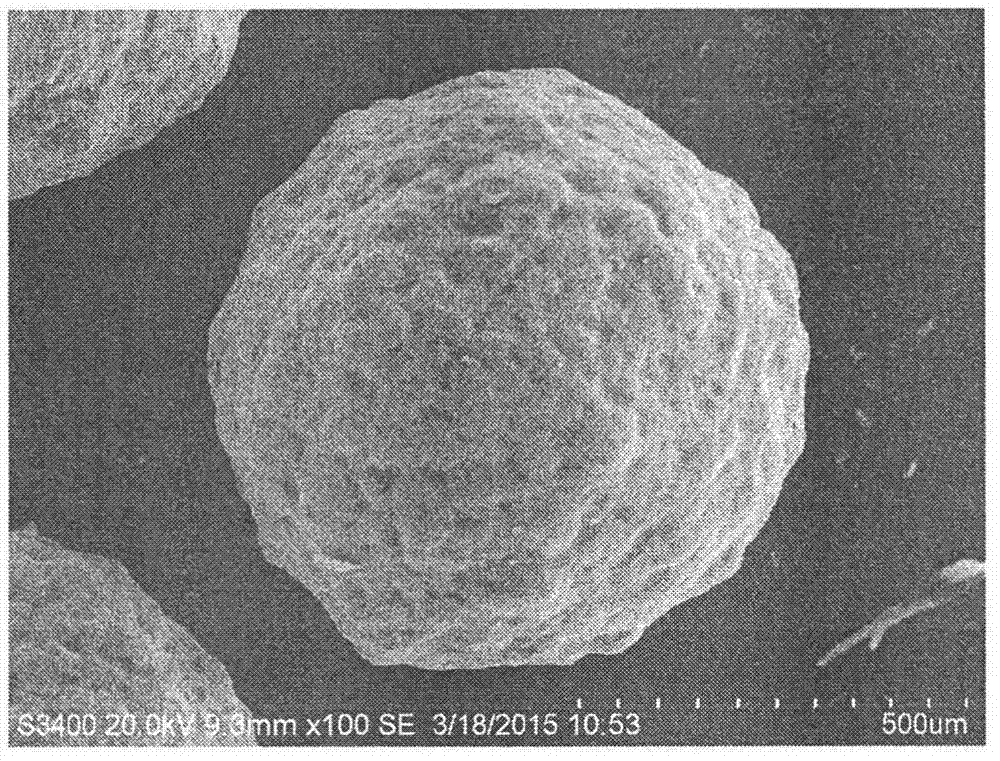
Development and application of cryptotanshinone sustained release solid dispersion pellet
A technology of solid dispersion and cryptotanshinone, applied in the field of medicine, can solve the problem of no published patent, and achieve the effect of simple and feasible technical operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of cryptotanshinone sustained-release solid dispersion pellets
[0023] Precisely weigh 0.1g of cryptotanshinone, 0.5g of ethyl cellulose and 0.1g of polyvinylpyrrolidone, dissolve them together in 30mL of ethyl acetate and 20mL of absolute ethanol, stir until clear, and wait for application. Take by weighing 5g of 25-30 mesh blank ball cores and add them in the fluidized bed hopper, turn on the blower fan and heating device to preheat for a period of time, and start to apply the medicine. The process conditions are: nozzle diameter: 0.5mm; fluidized bed temperature: 20-40°C; liquid spray rate: 0.5-5ml / min; spray gun spray pressure: 0.01-0.1Mpa; blast frequency: 15-35HZ. After adding the drug, the drug-loaded pellets were kept in a fluidized state and continued to boil for 60 minutes to obtain the final product.
[0024] (2) Determination of in vitro release of cryptotanshinone sustained-release solid dispersion pellets
[0025] Carry out according to ...
Embodiment 2
[0031] (1) Preparation of cryptotanshinone sustained-release solid dispersion pellets
[0032] According to the mass ratio of cryptotanshinone and ethyl cellulose: 1:1, 1:5, and 1:10, take 0.1 g of cryptotanshinone and appropriate amount of ethyl cellulose, and dissolve them in 30 mL of ethyl acetate and 20 mL of absolute ethanol In, stir until clarified, wait for the medicine. The sustained-release solid dispersion pellets of each prescription were prepared according to the method described in Example 1.
[0033] (2) Determination of in vitro release of cryptotanshinone sustained-release solid dispersion pellets: the determination method is the same as that described in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com