2-(alpha-hydroxy amyl) benzamide derivate as well as preparation method, preparation and application thereof
A technology of benzamide and hydroxypentyl group is applied in the field of medicine to achieve the effect of good in vitro stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
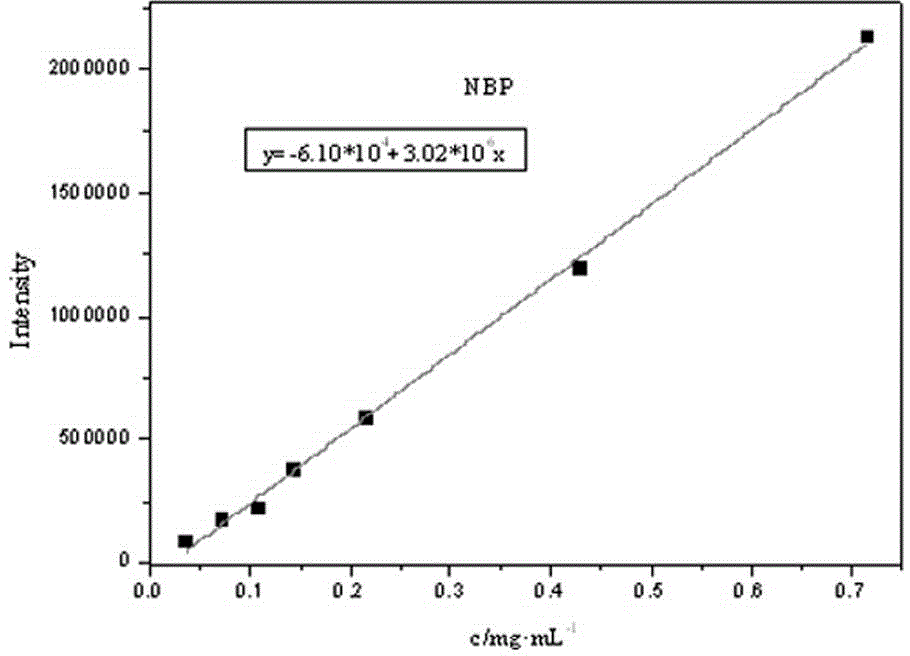
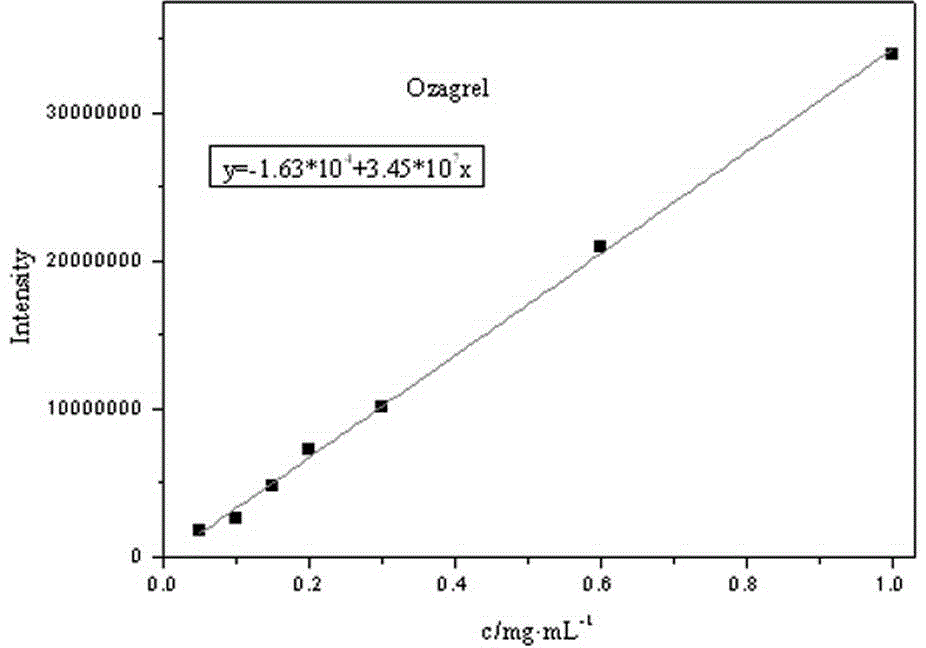
Image
Examples
preparation example Construction
[0063] The preparation method of 2-(α-hydroxypentyl) benzamide derivatives of the present invention comprises the following steps:
[0064] A. Dissolve the raw material butylphthalide in a solvent, adjust the pH value to 10~14 with alkali, add diamine with a reaction molar weight more than 1 times, then add a catalyst and stir the reaction for 24~48h at room temperature to obtain the intermediate product a;
[0065] B. Condensing the obtained intermediate product a with a carboxyl-containing compound under the condition of a condensing agent to obtain the target product.
[0066] The solvent described in the A step is a kind of in polar solvent or non-polar solvent.
[0067] The solvent described in step A is one of methanol, ethanol, dimethyl sulfoxide DMF, N-methylpyrrolidone NMP, dichloromethane, and tetrahydrofuran THF.
[0068] The base described in the A step is an inorganic base or an organic base.
[0069] The alkali described in step A is one of sodium hydroxide, po...
Embodiment 1
[0089] Example 1 (E)-N-(4-(3-(4-((1H-imidazolyl)methyl)phenyl)acrylamido)butyl)-2-(1-hydroxypentyl)benzyl Synthesis of Amide (Compound 1)
[0090]
[0091] (1) Synthesis of N-(4-aminobutyl)-2-(1-hydroxypentyl)benzoic acid
[0092] In a 50.0 mL round bottom flask, dissolve butylphthalide (0.19 g, 1 mmol) in 10 mL of dichloromethane, add diisopropylethylamine (0.44 mL, 2.5 mmol) and 4-dimethylaminopyridine (0.0366 g, catalytic amount), after stirring at room temperature for a while, 1,4-butanediamine (0.42 mL, 5 mmol) was added dropwise. After stirring at room temperature for more than 24 hours, after the reaction was detected by TLC, the white solid insoluble matter was removed by filtration, and the mother liquor was concentrated directly to obtain an oily substance, which was separated by silica gel column chromatography (dichloromethane:methanol=20:1) to obtain an oily substance (0.2380g , 90.1%)
[0093] 1 H NMR (600MHz, CDCl 3 )δ:8.34(s,1H),7.55(d,J=7.74Hz,1H),7.40...
Embodiment 2
[0103] Example 2 (E)-N-(2-(2-(3-(4-((1H-imidazolyl)methyl)phenyl)acrylamido)ethoxy)ethoxy)ethyl)- Synthesis of 2-(1-hydroxypentyl)benzamide (compound 2)
[0104]
[0105] (1) Synthesis of N-(2-(2-aminoethoxy)ethyl)-2-(1-hydroxypentyl)benzoic acid
[0106] In a 50.0 mL round bottom flask, dissolve butylphthalide (0.19 g, 1 mmol) in 10 mL of dichloromethane, add diisopropylethylamine (0.44 mL, 2.5 mmol) and 4-dimethylaminopyridine (0.0366 g, catalytic amount), after stirring at room temperature for a while, 1,2-bis(2-aminoethoxy)ethane (0.73 mL, 5 mmol) was added dropwise. TLC detection, stirring at room temperature for more than 24 hours. After the reaction, the mother liquor was concentrated directly to obtain an oil, which was separated by silica gel column chromatography (dichloromethane:methanol=20:1) to obtain an oil (0.2980g, 88.1%)
[0107] 1 HNMR (600MHz, CDCl 3 )δ:8.40(s,1H),7.58(d,J=7.77Hz,1H)), 7.43(m,1H),7.29(dd,J=7.66Hz,J=14.94Hz,2H),4.92(dd ,1H),3.56(m,6H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com