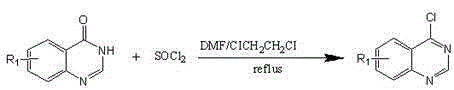Quinazoline-containing thioether substituted pentadiene ketone derivatives, and preparation method and application thereof
A kind of technology of pentadienone and oxazoline sulfide, which is used in pentadienone derivatives substituted with quinazoline sulfide and the fields of preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
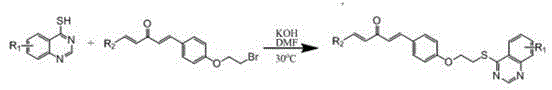
[0039] Example 1: Compound (1E,4E) 1-(2-thienyl)-5-(4-(2-((quinazoline)-4-thio)ethoxy)phenyl)-1,4 - Pentadien-3-one:
[0040] (1) quinazoline-4-(3 H )-ketone synthesis
[0041] Add anthranilic acid (0.2 mol) and formamide (0.8 mol) into a 250 mL three-neck flask equipped with a thermometer and a condenser tube to keep the temperature at 135-150 °C for reaction. During this process, the system changes from light gray turbid liquid to Yellowish-brown clear liquid, TLC tracking reaction (developer: petroleum ether: ethyl acetate = 1:1, V / V), the reaction is complete in about 5 hours, after natural cooling to 100°C, slowly add 1.0 times the volume of water to the system, Decompose the excess formamide, and at the same time, a light gray solid precipitates out. After natural cooling, transfer it to a large beaker, and then add 1.5 times the volume of water, and the off-white solid still precipitates. Filter it with suction and recrystallize it with absolute ethanol to obtain a ...
Embodiment 2
[0054] Example 2: Compound (1E, 4E) 1-(2-thienyl)-5-(4-(2-((8-methylquinazoline)-4-thio)ethoxy)phenyl) Synthesis of -1,4-pentadien-3-one:
[0055] (1) Intermediate 8-methylquinazoline-4-(3 H )-ketone preparation: the synthesis steps and process conditions are the same as in Example 1 (1), the difference is that 8-methylanthranilic acid is a raw material;
[0056] (2) Preparation of intermediate 8-methyl-4-chloroquinazoline: the synthesis steps and process conditions are the same as in Example 1 (2), the difference is that 8-quinazoline-4-(3 H )-ketone is a raw material;
[0057] (3) Preparation of intermediate quinazoline-4-thiol: the synthesis steps and process conditions are the same as in Example 1 (3), the difference is that 8-methyl-4-chloroquinazoline is used as the raw material;
[0058] (4) Preparation of intermediate (1E) 4-(4-hydroxyphenyl)-3-buten-2-one: the synthesis steps and process conditions are the same as in Example 1 (4);
[0059] (5) Preparation of inte...
Embodiment 3
[0062] Example 3: Compound (1E,4E) 1-(2-furyl)-5-(4-(2-((quinazoline)-4-thio)ethoxy)phenyl)-1,4 -Synthesis of pentadien-3-one:
[0063] (1) Intermediate quinazoline-4-(3 H )-ketone preparation: synthetic steps and process conditions are the same as embodiment one (1);
[0064] (2) Preparation of intermediate 4-chloroquinazoline: the synthesis steps and process conditions are the same as in Example 1 (2);
[0065] (3) Preparation of the intermediate quinazoline-4-thiol: the synthesis steps and process conditions are the same as in Example 1 (3);
[0066] (4) Preparation of intermediate (1E) 4-(4-hydroxyphenyl)-3-buten-2-one: the synthesis steps and process conditions are the same as in Example 1 (4);
[0067] (5) Preparation of intermediate 1-(4-hydroxyphenyl)-5-(2-furyl)-1,4-pentadien-3-one: the synthesis steps and process conditions are the same as in Example 1 (5 ), the difference is that furfural is a raw material;
[0068] (6) Preparation of intermediate 1-(4-(2-bromo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com