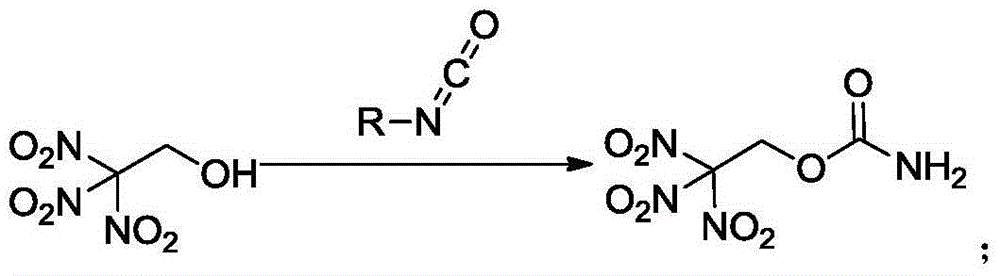
Synthesis method of trinitroethyl carbamate
A technology of trinitroethyl carbamate and synthesis method, which is applied to the preparation of carbamic acid derivatives, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of inconvenient use, unstable carbamoyl chloride, and unsuitable TNC scale-up synthesis and other problems, to achieve the effect of fast reaction rate, easy scale-up synthesis, and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Dissolve trinitroethanol (5.4318g, 0.03mol) in dichloromethane (20mL) in a 100mL three-neck flask equipped with a thermometer and magnetic stirring, and add chlorosulfonyl isocyanate (5.4 mL, 0.06mol), stirred and reacted at this temperature for 1h, removed the ice-water bath and returned to room temperature for reaction, TLC monitored the reaction, and the reaction of the raw materials was complete in about 2h, added 25mL of water dropwise to the reaction mixture and heated and stirred until no bubbles were generated, ethyl acetate After extraction, the combined organic phases were dried and concentrated to obtain 6.5861 g of white solid with a yield of 98.1%. Decomposition temperature: 1 H NMR (300MHz, [D 6 ]acetone): δ=6.78,6.49,5.70ppm; 13 C NMR (100MHz, [D 6 ]acetone): δ=154.31, 125.53, 61.60ppm; IR (KBrpellet): 3454, 3354, 3304, 3003, 2963, 2917, 1735, 1591, 1443, 1401, 1368, 1328, 1302, 1250, 1169, 1107, 1026,910,875,857,805,784,772,743,538.2cm -1 , elementa...
Embodiment 2
[0019] Dissolve trinitroethanol (5.4315g, 0.030mol) in dichloromethane (20mL) in a 100mL three-neck flask equipped with a thermometer and magnetic stirring, and add trichloroacetyl isocyanate (3.9 mL, 0.033mol), stirred and reacted at this temperature for 1h, removed the ice-water bath and returned to room temperature for reaction, TLC monitored the reaction, and the raw materials were completely reacted in about 3h, added 25mL water dropwise to the reaction mixture and heated and stirred until no bubbles were generated, ethyl acetate After extraction, the combined organic phases were dried and concentrated to obtain 6.3782 g of white solid with a yield of 95.0%.
Embodiment 3
[0021] Dissolve trinitroethanol (5.4312g, 0.03mol) in chloroform (20mL) in a 100mL three-necked flask equipped with a thermometer and magnetic stirring, cool to 0°C with an ice-water bath, and then add chlorosulfonyl isocyanate (5.4 mL, 0.06mol), stirred and reacted at this temperature for 0.5h, removed the ice-water bath and returned to room temperature for reaction, TLC monitored the reaction, and after 1.5h, the raw materials were completely reacted, and 25mL of water was added dropwise to the reaction mixture and heated and stirred until no bubbles were generated. Extracted with ethyl ester, combined organic phases were dried and concentrated to obtain 6.4517 g of white solid with a yield of 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com