Recombination human CYP3A4 (cytochrome P450 3A4) /CPR (cytochrome P450 oxidoreductase) /cyt b5 (cytochrome b5) protein co-transfection co-expression method
A CYP3A4, co-transfection technology, applied in the field of biomedicine, can solve the problems of low activity, difficult hydrolysis, and difficult acquisition of expression vectors, and achieve the effect of high cell culture density, maintaining enzyme biological activity, and easy enzyme biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
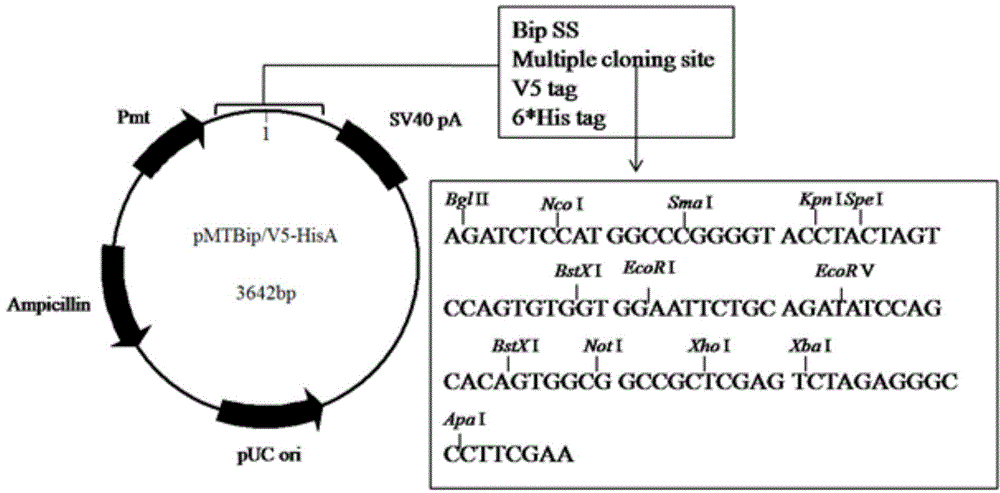
Image
Examples
Embodiment 1
[0079] Example 1 Amplification of CYP3A4, CPR and cyt b5 cDNA and Construction of Recombinant Plasmid
[0080] The sequences of CYP3A4, CPR, and cyt b5 cDNA fragments without removing the start codon and stop codon are SEQ ID NO.1, SEQ ID NO.2, and SEQ ID NO.3, respectively.
[0081] 1.1 Amplification of CYP3A4 cDNA
[0082] First design the following CYP3A4 cDNA primers, shown in SEQ ID NO.4 to SEQ ID NO.11, including the following:
[0083] F: CTAGTCTAGAACCATGGCTCTCATCCCAGACTTGGCCATGGAAACCTGGCT (SEQ ID NO. 4)
[0084] R: CCGCTCGAGGGCTCCACTTACGGTGCCATCCCTTGACTCAACCT (SEQ ID NO. 5)
[0085] P1: CTAGTCTAGAACCATGGCTCTCATCCCAGACTTGGC (SEQ ID NO. 6)
[0086] P2: AAATCTGAGGCGGGAAGCAGCTGCTTCCCGCCTCAGATTTCTCAC (SEQ ID NO. 7)
[0087] P3: CTGCTTCCCGCCTCAGATTTCTCACAAATCTGAGGCGGGAAGCAG (SEQ ID NO. 8)
[0088] P4: CCACCTATGATACTGTGCTATAGCACAGTATCATAGGTGG (SEQ ID NO. 9)
[0089] P5: TAGCACAGTATCATAGGTGGCCACCTATGATACTGTGCTA (SEQ ID NO. 10)
[0090] P6: CCGCTCGAGGGCTCCACTTACGGTGCCATC...
Embodiment 2
[0108] Example 2 The recombinant expression plasmid was transfected into Drosophila S2 cells and screened to obtain a stable monoclonal cell line.
[0109] Resuscitate Drosophila S2 cells with complete medium (Schneider’s Insect Medium plus 10% FBS) at 28°C without CO 2 cultured in an incubator for several days. When the cells are growing well, spread a 6-well plate, 3*10 per well 6 cells in a total volume of 3 ml and cultured for 1 day.
[0110] Calcium phosphate transfection method The recombinant expression vectors inserted into CYP3A4, CPR, and cyt b5 cDNA respectively and the selection plasmid pCoblast were co-transfected into S2 cells at a certain ratio, that is, the mass ratio was 6 μg: 6 μg: 6 μg: 1 μg. After culturing for 24 hours, replace with fresh complete medium and culture for 2 days. In other embodiments, the ratio of the recombinant expression vector CYP3A4:CPR:cyt b5 cDNA:selection plasmid can be any specific value within the range of 5-8:5-8:5-8:1.
[011...
Embodiment 3
[0114] Example 3 Preparation of monoclonal stably transfected cell microsomes co-expressing CYP3A4 / CPR / cyt b5
[0115] Large-scale culture of CYP3A4 / CPR / cyt b5 monoclonal stable cell line G3, and CuSO 4 (final concentration 750 μM) induced for 4 days.
[0116] The cells were collected by centrifugation at 3000rmp for 5min, and sucrose / Tris buffer (Tris 50mM; MgCl 2 3mM; sucrose 200mM, pH7.4), sonication, power 100W, ultrasonic 5s, interval 10s, 10 cycles. Centrifuge at 10,500 g for 25 min at 4°C after sonication. Transfer the supernatant to an ultracentrifuge tube, place in an ultracentrifuge, centrifuge at 105,000 g for 1 h at 4°C, and carefully discard the supernatant.
[0117] The precipitate was resuspended in 0.1M potassium phosphate buffer (pH 7.4) to obtain the CYP3A4 / CPR / cyt b5 microsomal solution, the protein concentration was determined by BCA method, and stored at -80°C for future use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com