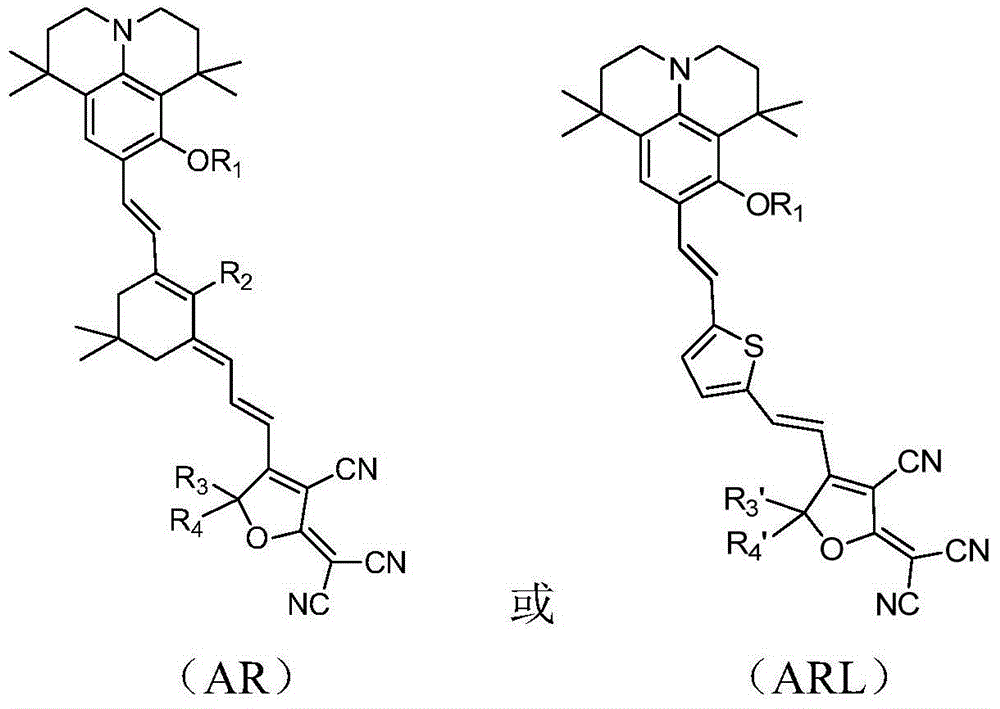
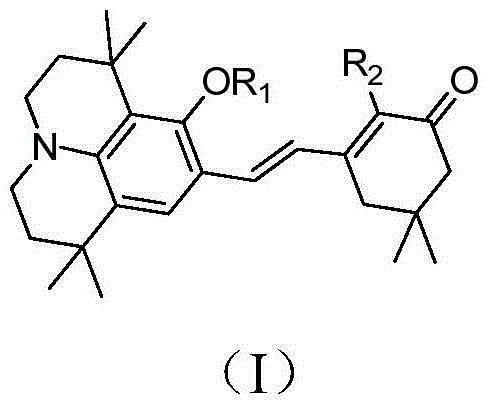
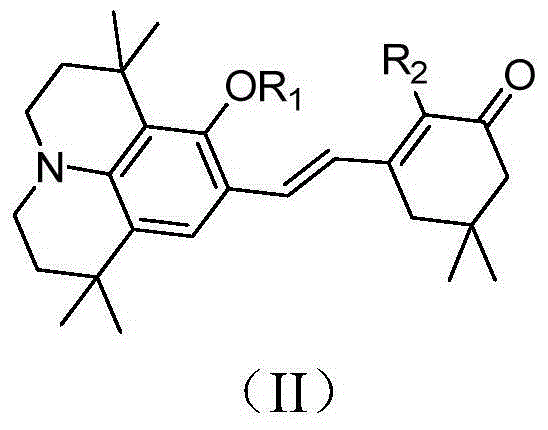
Organic second-order nonlinear optical chromophore with D-pi-A structure and its synthesis method and use
A technology of second-order nonlinearity and synthesis method, which is applied in the field of organic second-order nonlinearity and can solve the problems of low solubility, large intermolecular interaction force, and small electro-optic coefficient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] An organic second-order nonlinear optical chromophore with a D-π-A structure was synthesized as shown below:
[0127]
[0128] The synthetic route is as follows:
[0129]
[0130] 1) Synthesis of compound 1 represented by 1 in the formula
[0131] Add 2.72g (0.01mol) 8-hydroxyl-1,1,7,7-tetramethyljulonidine-9-carbaldehyde, 2.05g (0.015mol) 6-chloro-1-hexyl to a 50mL glass three-neck flask Alcohol and 30mL redistilled N,N-dimethylformamide, in N 2 Add 1.7g (0.012mol) of dried anhydrous potassium carbonate under protection, react overnight at 120°C, cool down after the reaction, remove potassium carbonate by filtration, pour the filtrate into water to obtain a dark blue solution, and extract it three times with ethyl acetate , combine the organic phases, dry the combined organic phases with anhydrous magnesium sulfate overnight, filter, and remove ethyl acetate by rotary evaporation, and the residue is separated by column chromatography (using 200-300 mesh silica ...
Embodiment 2
[0154] An organic second-order nonlinear optical chromophore with a D-π-A structure was synthesized as shown below:
[0155]
[0156] The synthetic route is as follows:
[0157]
[0158] Compound 1 represented by 1 in the formula, compound 2 represented by 2 in the formula, compound 3 represented by 3 in the formula, compound 4 represented by 4 in the formula and compound 5 represented by 5 in the formula Synthesis and embodiment 1 same.
[0159] 1) Synthesis of compound 6 shown in the formula
[0160] Dissolve 5.4g (75mmol) ethyl vinyl ether in 25ml redistilled tetrahydrofuran under N 2 Cool to -85°C under protection, add 38.5ml (1.3M, 50mmol) tert-butyllithium n-hexane solution dropwise; After keeping at -15°C for 30-60 minutes, cool down to -80°C, add dropwise 5ml of THF solution in which 2.8g (25mmol) trifluoroacetophenone was dissolved at this temperature, and keep at this temperature for 40 minutes after the dropwise addition , then allowed to heat up naturally...
Embodiment 3
[0169] An organic second-order nonlinear optical chromophore with a D-π-A structure was synthesized as shown below:
[0170]
[0171] The synthetic route is as follows:
[0172]
[0173] 1) Synthesis of compound 3 shown in the formula
[0174] Dissolve 1 equivalent of compound 1 and 1.5 equivalents of compound 2 in an appropriate amount of redistilled DMF, and add 1.2 equivalents of K 2 CO 3 (dried in a muffle furnace for more than 4 hours), at N 2 Stir the reaction overnight at 110°C under protection; 2 Cool to room temperature under protection, pour it into deionized water and stir, the mixture is extracted with ethyl acetate, the organic phases are combined, washed with saturated NaCl, anhydrous MgSO 4 The combined organic phases were dried overnight, filtered, and ethyl acetate was removed by rotary evaporation, purified by column chromatography (the volume ratio of petroleum ether: acetone was 400:50), and a yellow solid was obtained after drying with a yield of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electro-optic coefficient | aaaaa | aaaaa |
| electro-optic coefficient | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com