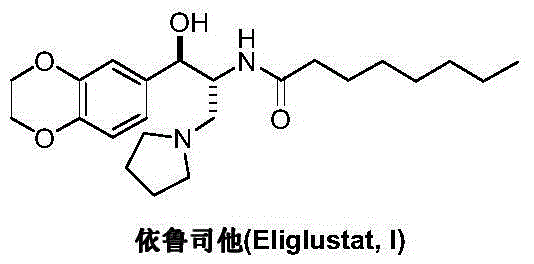
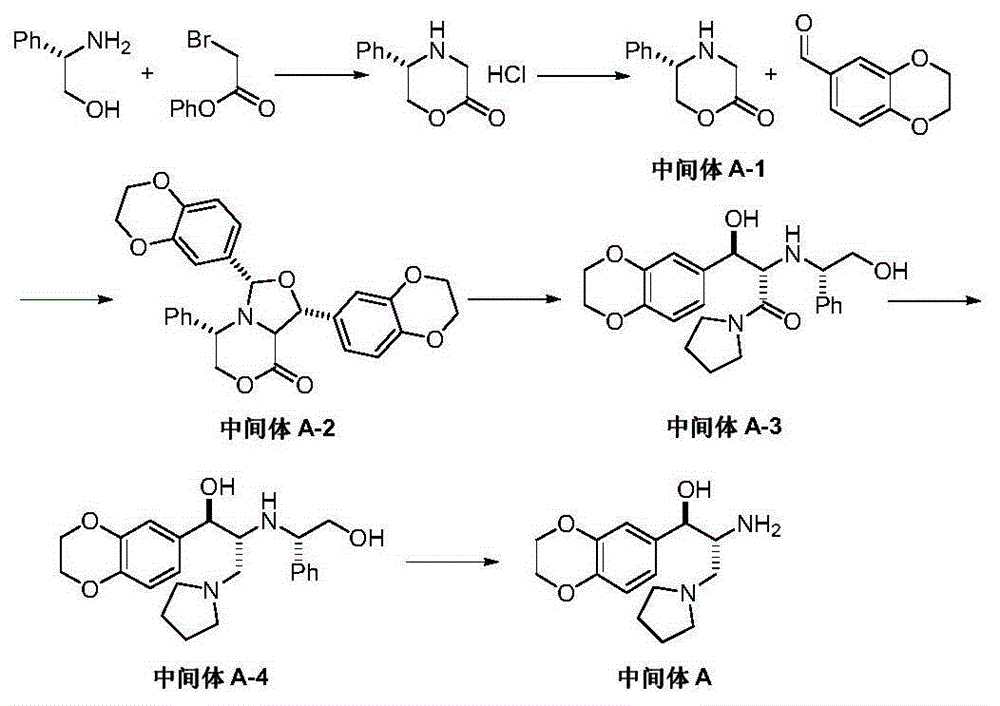
Preparation method of eliglustat
A technology of ebrustat and preparation steps, which is applied in the field of preparation of ebrustat, a drug for treating type I Gaucher disease, can solve problems such as expensive reduction catalysts, difficulty in obtaining raw materials, and numerous reaction steps, so as to promote development and facilitate the development of raw materials. The effect of gain, process, environmental protection and economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 0-5°C under a nitrogen atmosphere, add pyrrolidine (7.1g, 0.1mol), cuprous bromide (0.72g, 5mmol), triethylamine (2.0g, 0.2mmol) and 50mL of tetrahydrofuran into a reaction flask, stir 1-Bromo-2-nitroethane (18.5 g, 0.12 mmol) was added dropwise. After the drop was completed, the temperature was slowly raised to 30-35° C., stirred for 36 hours, and the reaction was detected by TLC. Cool to room temperature, remove insoluble matter by filtration, recover the solvent under normal pressure, and distill under reduced pressure to obtain 11.8 g of light yellow liquid 2-(pyrrolidin-1-yl)-1-nitroethane (III), with a yield of 81.9%;1 H NMR (CDCl 3 )δ1.72(m, 4H), 2.47(m, 4H), 2.78(t, 2H), 4.70(t, 2H); EI-MS m / z: 145[M+H] + .
Embodiment 2
[0043] Under a nitrogen atmosphere and at -5-0°C, add 2-nitro-1-(1-pyrrolidinyl)ethanone (7.9g, 0.05mol) and 50mL of tetrahydrofuran into the reaction flask, and add lithium aluminum hydride (2.7g , 0.15mol), the addition was completed, the temperature was raised to 35-45° C., and the reaction was stirred for 6 hours, and the reaction was detected by TLC. Add 5 mL of water dropwise to quench the reaction, add 25 mL of 15% sodium hydroxide solution, stir for 15 minutes, and filter to remove insoluble matter. It was extracted three times with dichloromethane, and the organic phases were combined and concentrated to obtain a brown-yellow oil. Distilled under reduced pressure to obtain 6.2 g of light yellow liquid 2-(pyrrolidin-1-yl)-1-nitroethane (III), yield 86.1%; 1 H NMR (CDCl 3 )δ1.72(m, 4H), 2.47(m, 4H), 2.76(t, 2H), 4.70(t, 2H); EI-MS m / z: 145[M+H] + .
Embodiment 3
[0045] At room temperature and under a nitrogen atmosphere, copper acetate monohydrate (0.20 g, 1 mmol), (1S, 3R, 7R)-1-phenyl-3-(1-benzotriazolyl)-1H was added to the reaction flask, 6H-Naphtho[1,2-e]piperidino[2,1-b][1,3]oxazine (VI) (0.43g, 1mmol) and 15mL tetrahydrofuran, heated to 50-60°C, stirred React for 3 hours until the solution is clear. Cool down to room temperature, add 2,3-dihydro-1,4-benzodioxanyl-6-aldehyde (II) (1.64g, 10mmol), stir for 30 minutes, cool down to -20°C, and slowly add 2-(Pyrrolidin-1-yl)-1-nitroethane (III) (2.12g, 15mmol) in THF (15mL) and 1,4-diazabicyclo[2.2.2]octane Tetrahydrofuran (15 mL) solution was kept at -20°C and stirred for 24-28 hours, and the reaction was detected by TLC. The reaction was quenched with 5 mL of saturated ammonium chloride at low temperature, raised to room temperature, extracted three times with dichloromethane, and the organic phases were combined and dried over anhydrous magnesium sulfate. Concentrate to give l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com