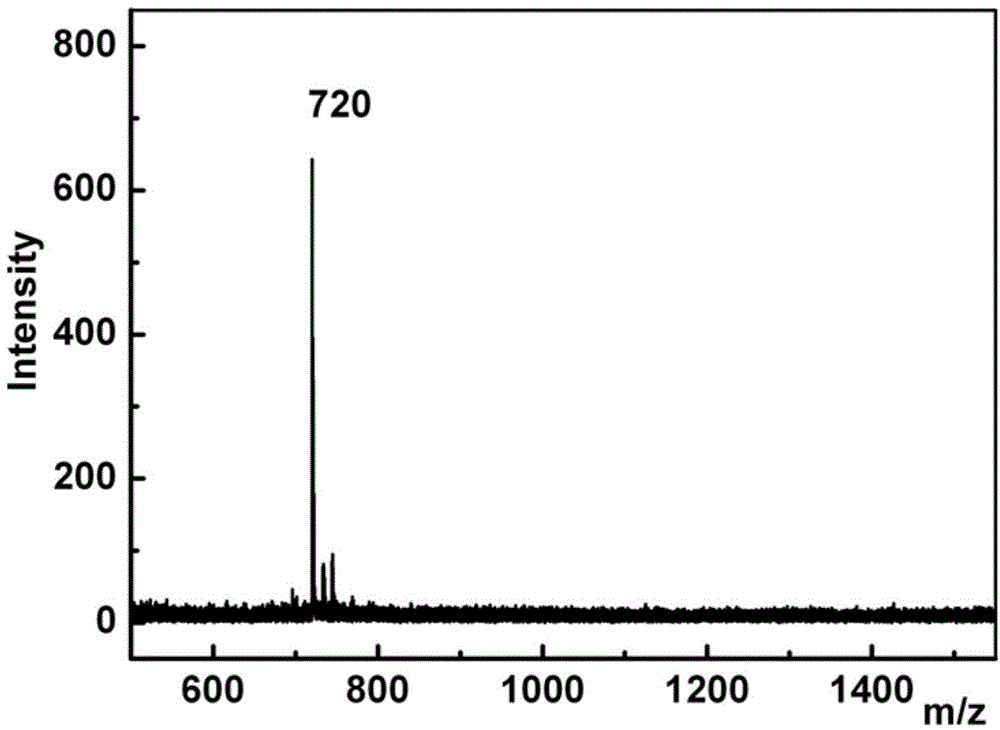
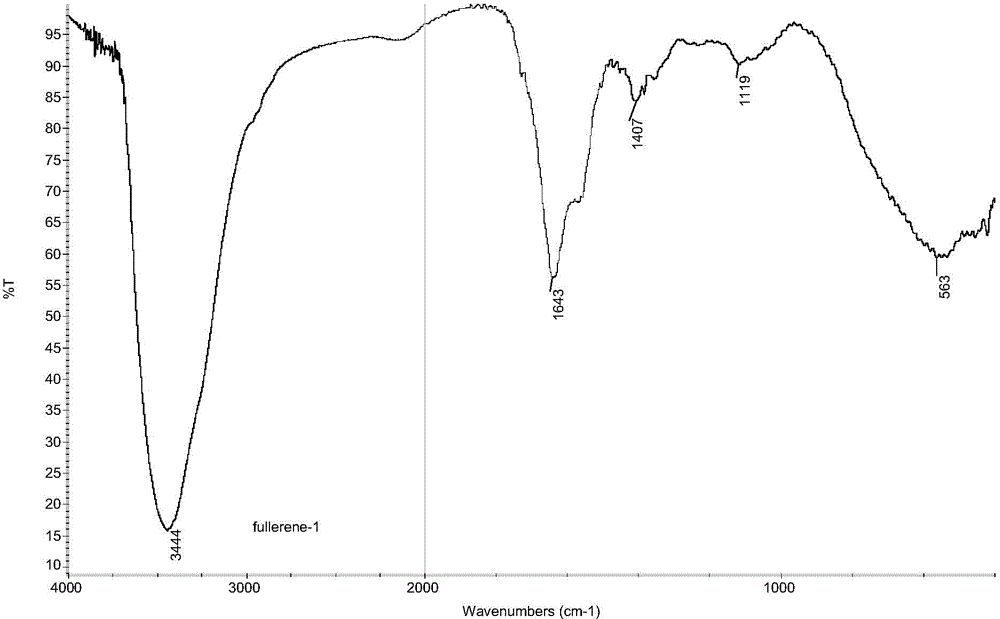
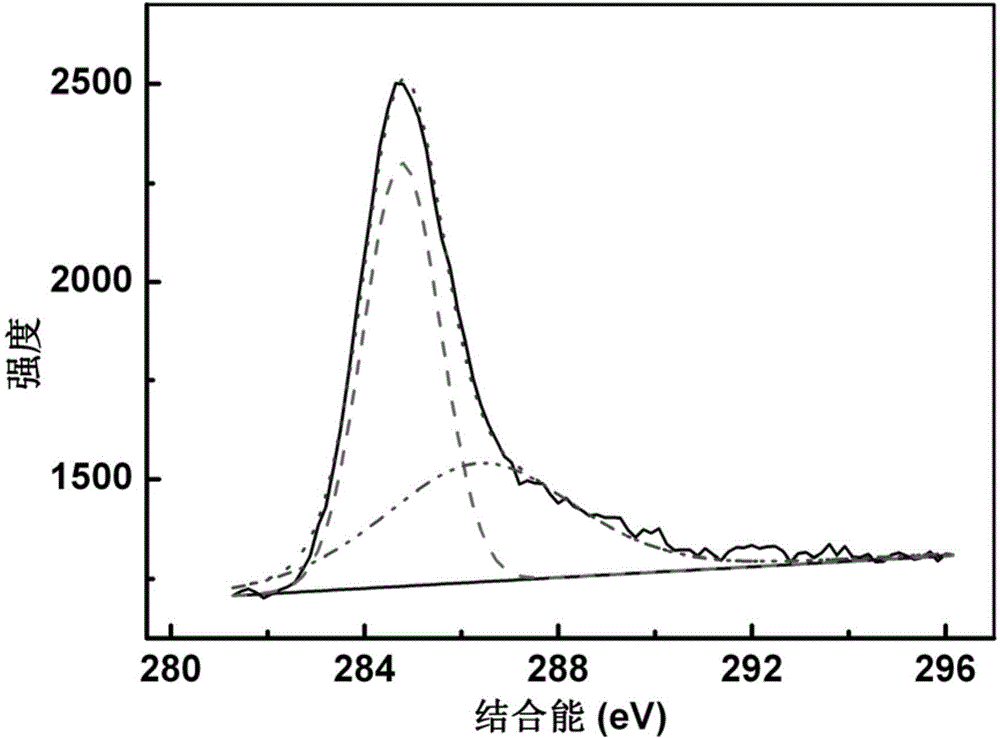
Preparation method of fullerol
A technology of fullerenols and fullerenes, which is applied in the field of liquid-phase reaction for the preparation of fullerenols, can solve problems such as environmental pollution, difficult operation, and easy volatility, so as to reduce the consumption of organic solvents, reduce the impact of environmental pollution, and purify The effect of the simplicity of the separation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Saturated C 60 Preparation of water-soluble fullerenol 1 with 2.5mol / L NaOH solution
[0046] (1) Dissolve 5.40g C in 200mL o-dichlorobenzene 60 , 300mL 2.5mol / L NaOH solution and 0.5mL 40% TBAH were fed into the reactor respectively, layered, the lye was in the upper layer, and the o-dichlorobenzene phase was in the lower layer;
[0047] (2) There is no need to isolate the air when the reaction system is exposed, and it is continuously stirred at room temperature for 60 hours, and the reaction is stopped;
[0048] (3) liquid separation, the organic solvent phase is washed with water and recovered for use, and the aqueous phase is dried under reduced pressure to obtain solidified fullererol thick product;
[0049] (4) Wash the crude fullererol product with ethyl acetate and methanol, redissolve it in deionized water, dialyze it with a dialysis bag with a molecular weight cut-off of 500-10000 Daltons, and dry it to obtain a solid pure product, which is fulle...
Embodiment 2
[0058] Example 2: Supersaturated C 60 Preparation of water-soluble fullerenol 2 with 2.5mol / L NaOH solution
[0059] (1) Disperse 5.40g C in 100mL o-dichlorobenzene 60 , 300mL 2.5mol / L NaOH solution and 0.5mL 40% TBAH were fed into the reactor respectively, layered, the lye was in the upper layer, and the o-dichlorobenzene phase was in the lower layer;
[0060](2) There is no need to isolate the air when the reaction system is exposed, and it is continuously stirred at room temperature for 72 hours, and the reaction is stopped;
[0061] (3) liquid separation, the organic solvent phase is washed with water and recovered for use, and the aqueous phase is dried under reduced pressure to obtain solidified fullererol thick product;
[0062] (4) Wash the crude fullererol product with ethyl acetate and methanol, redissolve it in deionized water, dialyze it with a dialysis bag with a molecular weight cut-off of 500-10000 Daltons, and dry it to obtain a solid pure product, which is f...
Embodiment 3
[0063] Example 3: Saturated C 60 Preparation of Fullerenol 3 with 0.5mol / L NaOH Solution
[0064] (1) Dissolve 0.54g C in 20mL o-dichlorobenzene 60 , 60mL 0.5mol / L NaOH solution and 0.05mL 40% TBAH were fed into the single-necked flask respectively, and the layers were separated, the lye was in the upper layer, and the o-dichlorobenzene phase was in the lower layer;
[0065] (2) There is no need to isolate the air when the reaction system is exposed, and it is continuously stirred at room temperature for 72 hours, and the reaction is stopped;
[0066] (3) liquid separation, the organic solvent phase is washed with water and recovered for use, and the aqueous phase is dried under reduced pressure to obtain solidified fullererol thick product;
[0067] (4) Wash the crude fullererol product with ethyl acetate and methanol, redissolve it in deionized water, dialyze it with a dialysis bag with a molecular weight cut-off of 500-10000 Daltons, and dry it to obtain a solid pure prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com