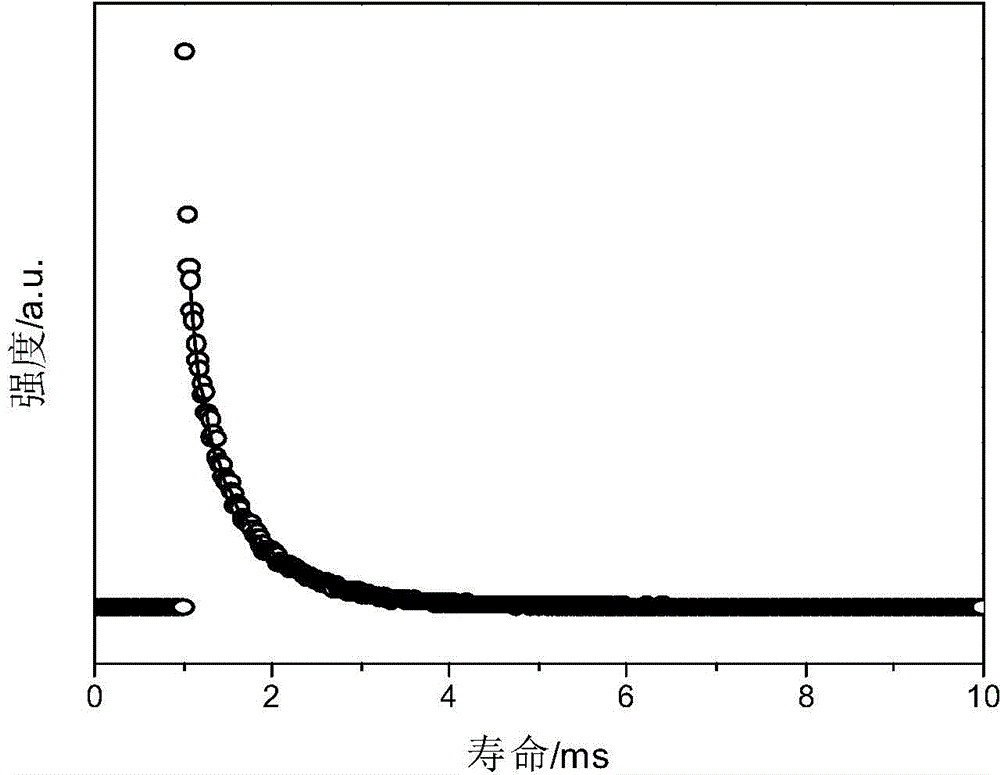
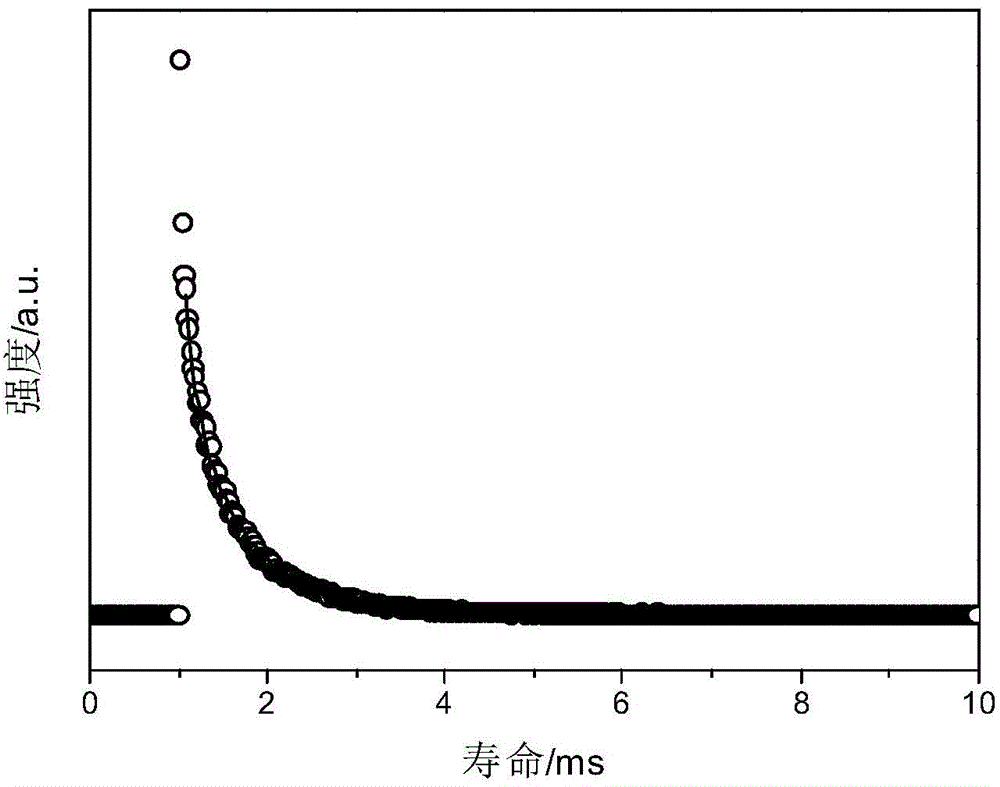
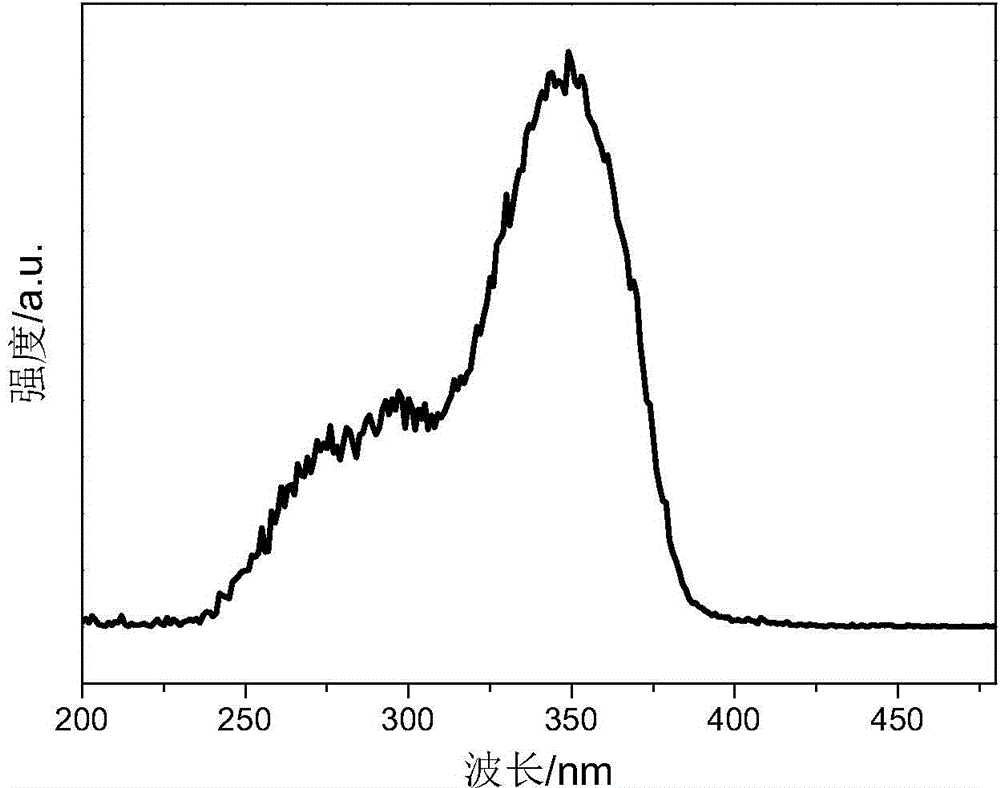
Caged oligomeric silsesquioxane prepared with TpySi as angle supplementing body, and rare earth luminescent material thereof
A technology of polysilsesquioxane and luminescent materials, applied in luminescent materials, silicon organic compounds, chemical instruments and methods, etc., can solve the problems of rare research reports and achieve good luminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] ①. Dissolve 444.4mg (2.0mmol) of α-thienoyltrifluoroacetone (commercially available) in 20mL of tetrahydrofuran, add 48.0mg (2mmol) of sodium hydride (commercially available), and heat and stir the mixed solution at 65°C for 1.5h Then 371 μL (2 mmol) of 3-chloropropyltriethoxysilane (commercially available) was added dropwise, and heated and stirred at 65° C. for 18 h under the protection of an inert gas to obtain a yellow solution.
[0057] ②, the yellow solution was suspended and evaporated to remove the solvent to obtain a yellow oil, which was dissolved in ether, filtered to remove the filter residue, then the filtrate was suspended to remove the ether, and dried at 70°C to obtain a yellow oil, which was TTASi.
[0058] ③, 402.5 mg (0.505 mmol) of 1,3,5,7,9,11,14-heptaisobutyltricyclo[7.3.3.15,11]heptasiloxane-3,7,14-tri Alcohol (T) was dissolved in 30 mL of chloroform (99.5%), 229.0 mg (0.527 mmol) TTASi was dissolved in 2 mL of tetrahydrofuran (99.5%), protected b...
Embodiment 2
[0070] ① Dissolve 4.0g (25.6mmol) of 2,2'-bipyridine (commercially available) in 30mL of glacial acetic acid, add 5.5mL of 30% hydrogen peroxide, heat and stir in an oil bath at 75°C for 3 hours, then add 4mL of 30% hydrogen peroxide, and continue stirring 10h. After stopping the reaction, the mixture was cooled to room temperature, and 100 mL of acetone was added to precipitate white crystals, which were filtered to obtain 2,2-bipyridine-N,N'-dioxide.
[0071] ②, add 3.7g (18.0mol) of 2,2'-bipyridine-N,N'-dioxide, add 18mL of 98% concentrated sulfuric acid in an ice-water bath, heat to 95°C until completely dissolved, then add 6.5mL of fuming nitric acid, Heat to reflux at 95°C for 20h. After the reaction was over, the mixture was cooled to room temperature, poured into 50mL of ice, and stirred constantly, and brown-red gas escaped, and the solution turned light green. Continue stirring until no brown-red gas was produced, and the solution turned light yellow. There was a l...
Embodiment 3
[0086] ①, Add 300.0mg (1.12mmol) 4'-chloro-2,2':6',2"-terpyridine (commercially available) and 2.16mL 1,3-propanediamine (commercially available) into the reaction flask at the same time, Heat and reflux at 120°C for 12h, then cool it down to room temperature, add 25mL double distilled water to produce a white precipitate, centrifuge, and dry at 70°C to obtain a white powder, which is designated as TpyNH 2 .
[0087] ②, 120.0mg (0.39mmol) TpyNH 2 Dissolve in 6 mL of ethanol, then add 160 μL (0.60 mmol) of γ-isocyanatopropyltriethoxysilane (ICPTES), stir and heat in an oil bath at 60°C for 40 hours to obtain a yellow solution. The solvent was removed by suspension evaporation, washed with n-hexane, centrifuged, and dried at 70°C to obtain a yellow oil, which was designated as TpySi.
[0088] ③, 402.5 mg (0.505 mmol) of 1,3,5,7,9,11,14-heptaisobutyltricyclo[7.3.3.15,11]heptasiloxane-3,7,14-tri Alcohol (T) (commercially available) was dissolved in 30 mL of chloroform (99.5%), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com