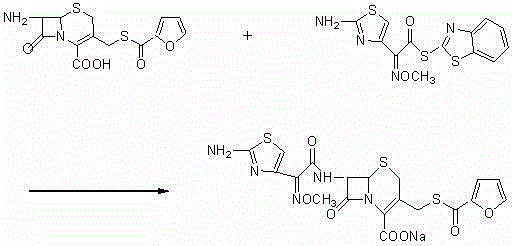
New preparation method of ceftiofur sodium
A technology for ceftiofur sodium and ceftiofur, which is applied in the direction of organic chemistry and the like, can solve the problems of product purity and solubility that cannot meet medicinal requirements, and the cost is difficult to control, and achieves shortened synthesis steps, low cost and simplified operation. effect of the program
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
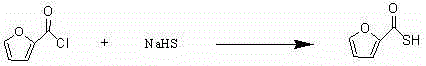
[0028] (1) Synthesis of intermediate (I) 2-furan methylthiol acid
[0029] Add 150 mL of distilled water to a clean 250 mL three-neck reaction flask equipped with a stirrer and a thermometer, then weigh 15.0 g (about 0.26 mol) of sodium hydrogen sulfide (NaHS), keep stirring and control the temperature at 25-27 °C Slowly add 25 mL (about 30 g, 0.23 mol) of furoyl chloride (furoyl chloride), keep stirring, and keep the temperature at 25-27 °C for 1 h. During this period, use 10% sodium hydroxide solution to adjust the pH at About 7.0, after the reaction is complete, use 10% hydrochloric acid to adjust the pH to about 1.5-2.0, cool to 5 ℃, crystallize for 1 h, filter under reduced pressure, wash with appropriate amount of water, get a light yellow solid by suction filtration, recrystallize with acetonitrile, and dry in vacuo 27 g of intermediate (I) was obtained.
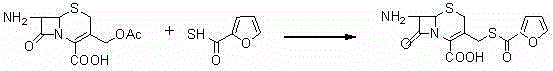
[0030] (2) Synthesis of intermediate (II) 7-amino-3-[(2-furyl-carbonyl)-thiomethyl]-3-cephem-4-carboxylic acid
...
Embodiment 2
[0036] (1) Synthesis of intermediate (I) 2-furan methylthiol acid
[0037] Add 150 mL of distilled water to a clean 250 mL three-neck reaction flask equipped with a stirrer and a thermometer, then weigh 15.0 g (about 0.26 mol) of sodium hydrogen sulfide (NaHS), keep stirring and control the temperature at 25-27 °C Slowly add 25 mL (about 30 g, 0.23 mol) of furoyl chloride (furoyl chloride), keep stirring, and keep the temperature at 25-27 °C for 1 h. During this period, use 10% sodium hydroxide solution to adjust the pH at About 7.0, after the reaction is complete, use 10% hydrochloric acid to adjust the pH to about 1.5-2.0, cool to 5 ℃, crystallize for 1 h, filter under reduced pressure, wash with appropriate amount of water, get a light yellow solid by suction filtration, recrystallize with acetonitrile, and dry in vacuo 26.7 g of intermediate (I) was obtained.
[0038] (2) Synthesis of intermediate (II) 7-amino-3-[(2-furyl-carbonyl)-thiomethyl]-3-cephem-4-carboxylic acid ...
Embodiment 3
[0044] (1) Synthesis of intermediate (I) 2-furan methylthiol acid
[0045] Add 150 mL of distilled water to a clean 250 mL three-neck reaction flask equipped with a stirrer and a thermometer, then weigh 15.0 g (about 0.26 mol) of sodium hydrogen sulfide (NaHS), keep stirring and control the temperature at 25-27 °C Slowly add 25 mL (about 30 g, 0.23 mol) of furoyl chloride (furoyl chloride), keep stirring, and keep the temperature at 25-27 °C for 1 h. During this period, use 10% sodium hydroxide solution to adjust the pH at About 7.0, after the reaction is complete, use 10% hydrochloric acid to adjust the pH to about 1.5-2.0, cool to 5 ℃, crystallize for 1 h, filter under reduced pressure, wash with appropriate amount of water, get a light yellow solid by suction filtration, recrystallize with acetonitrile, and dry in vacuo 28.2 g of intermediate (I) was obtained.
[0046] (2) Synthesis of intermediate (II) 7-amino-3-[(2-furyl-carbonyl)-thiomethyl]-3-cephem-4-carboxylic acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com