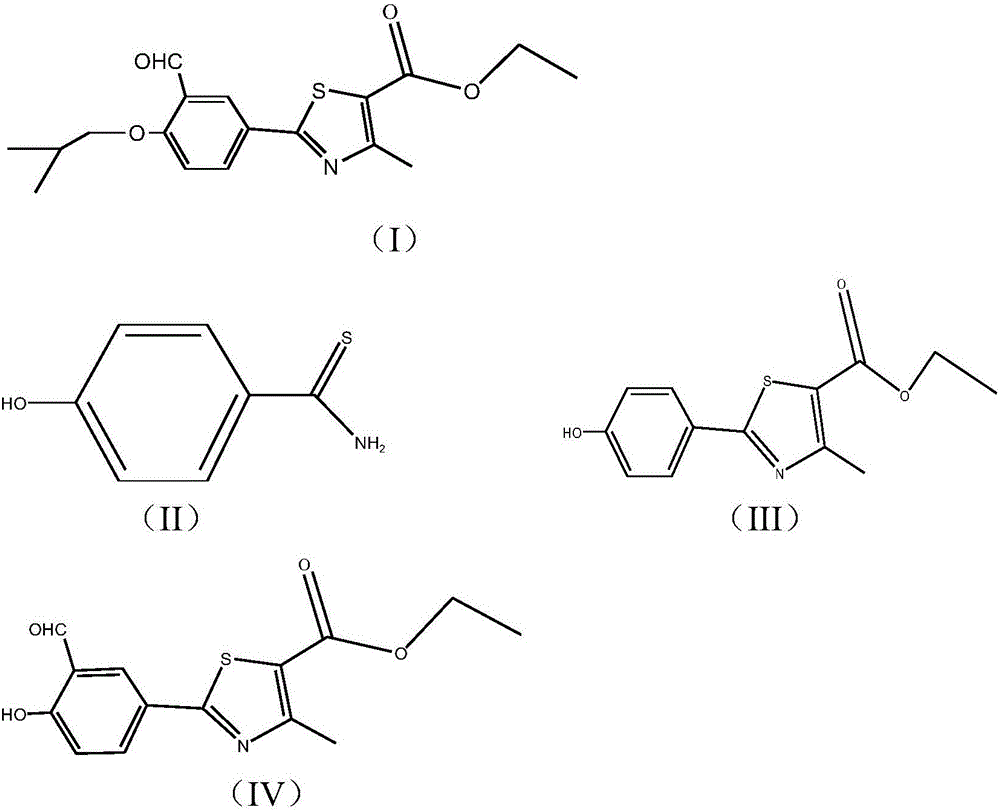
The synthetic method of 2‑(3‑formyl‑4‑isobutoxyphenyl)‑4‑methylthiazole‑5‑ethyl carboxylate
A technology of isobutoxyphenyl and ethyl thiazole formate is applied in the field of synthesis of high-purity ethyl 2--4-methylthiazole-5-carboxylate, and can solve the problems of various types of solvents, long reaction time and environmental pollution Large and other problems, to achieve the effect of short synthesis process and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Using p-cyanophenol and thioacetamide as starting materials, undergo sulfonylation reaction, continue thiazole reaction without separation, and then separate to obtain 2-(4-hydroxyphenyl)-4-methylthiazole-5 - ethyl carboxylate (formula III):
[0053] (1) Sulfurylation reaction: take p-cyanophenol and thioacetamide as starting raw materials, carry out sulphonylation reaction first, in a clean reaction bottle, drop into 70g polyphosphoric acid, 1.5g water, be mixed with Aqueous phosphoric acid system, add 12.7g thioacetamide and 20g p-cyanophenol into the system, stir and heat up to 40-80°C, and the reaction time is 2-8 hours; use HPLC to check whether the reaction is complete, and get 4-hydroxythiobenzene Formamide (formula II);
[0054] (2) Thiazolization reaction: After the sulfurylation reaction is complete, no separation is required, and the next step of thiazolization reaction is continued. 60 g of organic solvent ethanol is added to the reaction system to obtain a...
Embodiment 2
[0059] According to the various steps of Example 1, the thiazole reaction uses anhydrous phosphoric acid and methanol as the reaction solvent, and the rest of the process is the same as in Example 1 to obtain a white solid 2-(3-formyl-4-isobutoxyphenyl)-4 -Methylthiazole-5-ethyl carboxylate (formula I) 30.1 g, HPLC content 99.2%, yield 51.6%.
Embodiment 3
[0061] According to the various steps of Example 1, the formylation reaction uses polyphosphoric acid and phosphoric acid as the reaction solvent, the extraction solvent is ethyl acetate, and the rest of the process is the same as in Example 1 to obtain a white solid 2-(3-formyl-4-iso Butoxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester (Formula I) 28.9g, HPLC content 99.12%, yield 49.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com