Preparation method of manganese oxide ion sieve adsorbent and precursor thereof
An ion sieve and adsorbent technology, which is applied in the field of preparation of manganese-based ion sieve adsorbent and its precursor, can solve the problems of incomplete recovery of ion sieve, reduced cycle performance, attenuation of adsorption capacity, etc., so as to improve lithium adsorption performance, The effect of reducing dissolution loss and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
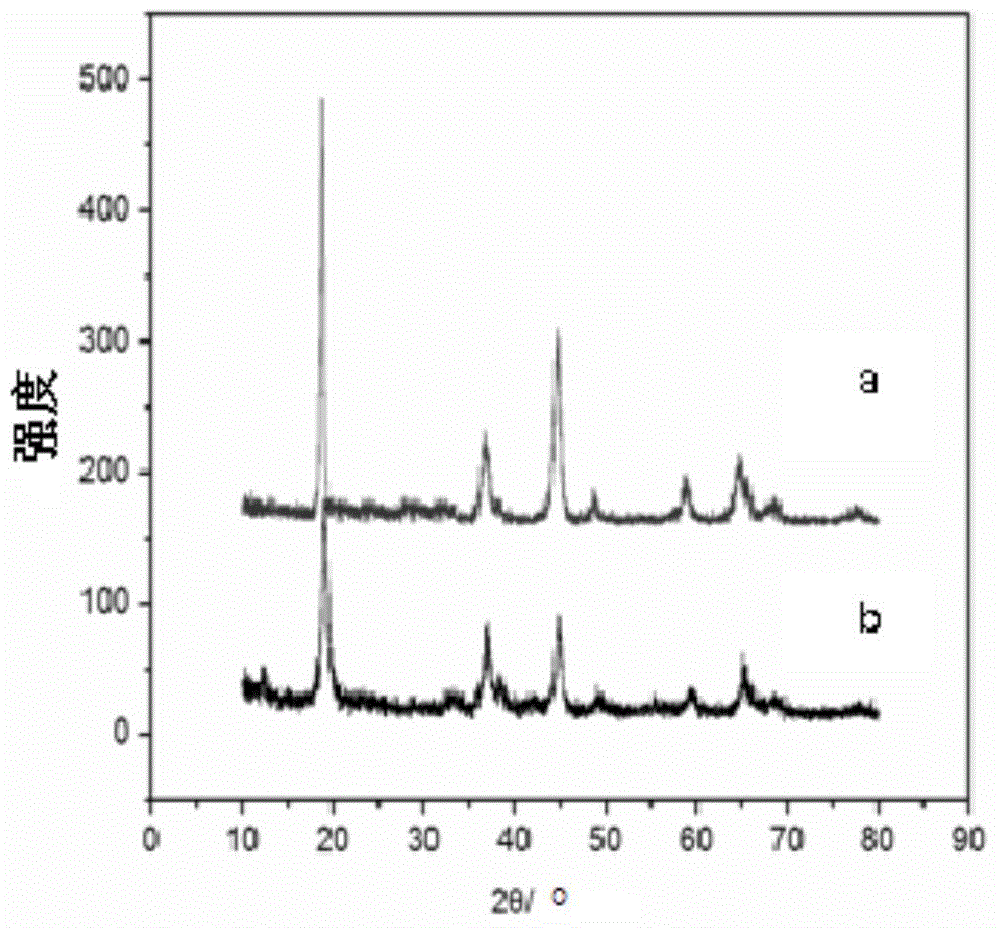
[0037] according to figure 1 The process shown is for the preparation of lithium ion sieve adsorbent and its precursor, the steps are as follows:
[0038] (1) Weigh lithium hydroxide (LiOH·H 2 O) 1.6886g, manganese acetate (Mn(CH 3 COO) 2 4H 2 (2) 12.2550g, dissolved in water respectively to obtain a lithium hydroxide solution and a manganese acetate solution; the lithium hydroxide solution was poured into the manganese acetate solution, and reacted in a constant temperature water bath at 80°C for 3h to generate a brown colloidal precipitate;
[0039] (2) Dry the colloidal precipitate in an oven at 120°C to obtain a light yellow solid; grind the solid into powder and put it in a muffle furnace at a rate of 5°C / min to 450°C for 12 hours to obtain intermediate Product 1 is Li 4 mn 5 o 12 powder;
[0040] (3) Will Li 4 mn 5 o 12 The powder is immersed in 500mL, 0.5mol / L hydrochloric acid solution, stirred for 5h to remove lithium ions, and filtered to obtain the interm...
Embodiment 2
[0046] according to figure 1 The process shown is for the preparation of lithium ion sieve adsorbent and its precursor, the steps are as follows:
[0047] (1) Weigh lithium hydroxide (LiOH·H 2 O) 1.2585g, manganese acetate (Mn(CH 3 COO) 2 4H 2 (2) 12.2550g, dissolved in water respectively to obtain lithium hydroxide solution and manganese acetate solution; the lithium hydroxide solution was poured into the manganese acetate solution, reacted in a constant temperature water bath at 90° C. for 2 hours, and produced a brown colloidal precipitate;
[0048] (2) Dry the colloidal precipitate in an oven at 120°C to obtain a light yellow solid; grind the solid into powder and put it in a muffle furnace at a rate of 5°C / min to 300°C for 18 hours to obtain intermediate Product 1 is Li 4 mn 5 o 12 powder;
[0049] (3) Will Li 4 mn 5 o 12 Add the powder into 500mL, 0.8mol / L hydrochloric acid solution, stir for 5h to remove lithium ions, and filter to obtain the intermediate pro...
Embodiment 3
[0054] according to figure 1 The process shown is for the preparation of lithium ion sieve adsorbent and its precursor, the steps are as follows:
[0055] (1) Weigh lithium hydroxide (LiOH·H 2 O) 1.8877g, manganese acetate (Mn(CH 3 COO) 2 4H 2 (2) 12.2550g, dissolved in water respectively to obtain lithium hydroxide solution and manganese acetate solution; Lithium hydroxide solution was poured into the manganese acetate solution, reacted in a constant temperature water bath at 70°C for 5h, and produced a brown colloidal precipitate;
[0056] (2) Dry the colloidal precipitate in an oven at 120°C to obtain a light yellow solid; grind the solid into powder and put it in a muffle furnace at a rate of 5°C / min to 500°C for 6 hours to obtain intermediate Product 1 is Li 4 mn 5 o 12 powder;
[0057] (3) Will Li 4 mn 5 o 12 Add the powder into 500mL, 0.8mol / L hydrochloric acid solution, stir for 5h to remove lithium ions, and filter to obtain the intermediate product 2, name...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com