Salidroside enteric-coated tablet and preparation method thereof
A technology of salidroside intestines and salidroside, which is applied in the direction of medical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. Solve the problems of poor absorption of tianoside, etc., to achieve the effect of increasing bioavailability, improving drug absorption, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
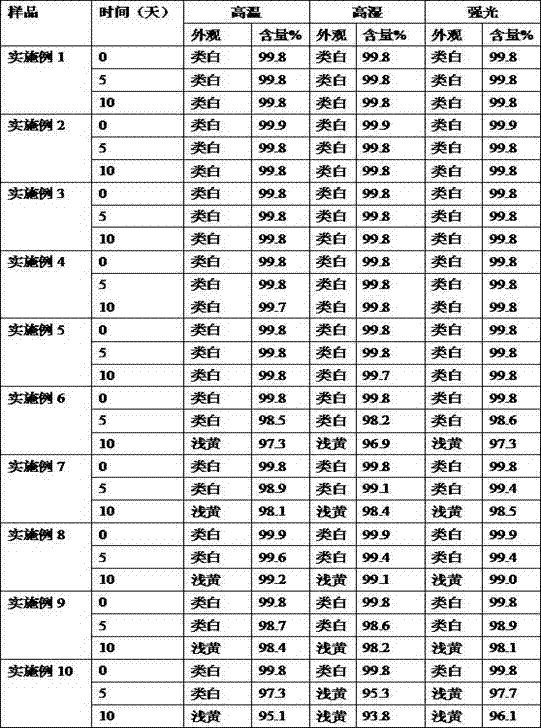
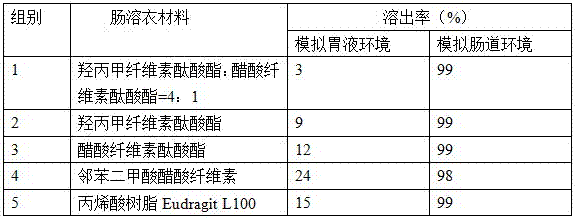
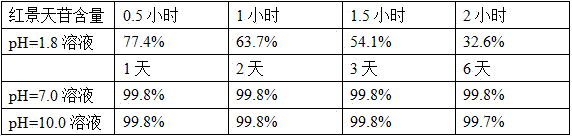
Examples
Embodiment 1
[0037] Salidroside enteric-coated tablets are made from the following components:
[0038] Proportion of original adjuvant (parts by weight)
[0039] 20 parts of salidroside
[0040] Hypromellose phthalate 8 parts
[0041] Cellulose acetate phthalate 2 parts
[0042] Microcrystalline cellulose 10 parts
[0043] 10% povidone K30 ethanol solution 10 parts
[0044] 20 parts of crospovidone
[0045] Magnesium stearate 5 parts
[0046] TPGS1000 5 copies
[0047] Sodium carbonate 2 parts
[0048] The preparation method is as follows:
[0049] (1) Weigh half the amount of cross-linked povidone, microcrystalline cellulose, TPGS1000, sodium carbonate, pulverize and mix, pass through an 80-mesh sieve, and set aside;
[0050] (2) Weigh the other half of the formula, crospovidone and magnesium stearate, pulverize and mix, pass through an 80-mesh sieve, and set aside;
[0051] (3) Weigh the formula amount of salidroside through an 80-mesh sieve for use;
[0052] (4) Add the salidroside obtained in step (3) an...
Embodiment 2
[0057] Salidroside enteric-coated tablets are made from the following components:
[0058] Proportion of original adjuvant (parts by weight)
[0059] 40 parts of salidroside
[0060] Hypromellose phthalate 10 parts
[0061] 10 servings of lactose
[0062] Hydroxypropyl cellulose 10 parts
[0063] Sodium Carboxymethyl Starch 10 parts
[0064] Magnesium stearate 5 parts
[0065] TPGS1000 5 copies
[0066] Sodium carbonate 1 part
[0067] The preparation method is as follows:
[0068] (1) Weigh half of the formula amount of sodium carboxymethyl starch, lactose, TPGS1000, sodium carbonate, pulverize and mix, pass through an 80-mesh sieve, and set aside;
[0069] (2) Weigh the other half of the formula amount, pulverize and mix the sodium carboxymethyl starch and magnesium stearate, pass through an 80-mesh sieve, and set aside;
[0070] (3) Weigh the formula amount of salidroside through an 80-mesh sieve for use;
[0071] (4) Add the salidroside obtained in step (3) and the mixture obtained in step (...
Embodiment 3
[0076] Salidroside enteric-coated tablets are made from the following components:
[0077] Proportion of original adjuvant (parts by weight)
[0078] 10 parts of salidroside
[0079] Cellulose acetate phthalate 10 parts
[0080] 20 parts of microcrystalline cellulose
[0081] Hypromellose 10 parts
[0082] Cross-linked povidone 10 parts
[0083] 5 servings of talcum powder
[0084] TPGS400 5 copies
[0085] Sodium bicarbonate 3 parts
[0086] The preparation method is as follows:
[0087] (1) Weigh half the amount of cross-linked povidone, microcrystalline cellulose, TPGS400, sodium bicarbonate, pulverize and mix, pass through an 80-mesh sieve, and set aside;
[0088] (2) Weigh the other half of the formula, crospovidone and talcum powder, pulverize and mix, pass through an 80-mesh sieve, and set aside;
[0089] (3) Weigh the formula amount of salidroside through an 80-mesh sieve for use;
[0090] (4) Add the salidroside obtained in step (3) and the mixture obtained in step (1) to a wet granulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com