Cabazitaxel long-circulation liposome injection and preparation method thereof
A long-circulation liposome and cabazitaxel technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problem of low drug loading, poor stability in vitro, and no effect and other issues, to achieve the effect of improving bioavailability, simplifying clinical use, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
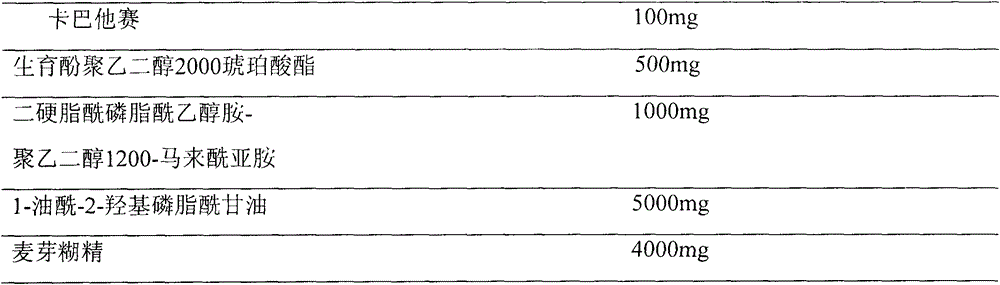
[0025] Prescription (100ml capacity)
[0026]
[0027] Preparation Process:
[0028] The prescribed amount of cabazitaxel, tocopheryl macrogol 2000 succinate, distearoylphosphatidylethanolamine-polyethylene glycol 1200-maleimide and 1-oleoyl-2-hydroxyphosphatidylglycerol Place in a 500ml round-bottomed flask, dissolve with 150ml of absolute ethanol, evaporate the ethanol on a rotary evaporator on a constant temperature water bath at 55-60°C at 100rpm and reduce pressure, so that the composition forms a uniform type at the bottom of the flask. Lipid film, add 6% maltodextrin aqueous solution into the above-mentioned round bottom flask, and wash the film with a rotary evaporator at 50°C until the lipid film is hydrated and becomes a white liposome suspension. 0.22 μm, membrane sizing (3 times each), the final dispersion was divided into vials, and then freeze-dried to obtain.
Embodiment 2
[0030] Prescription (100ml capacity)
[0031]
[0032] Prescribed amounts of cabazitaxel, tocopheryl polyethylene glycol succinate, distearoylphosphatidylethanolamine-polyethylene glycol 1200-maleimide and 1-oleoyl-2-hydroxyphosphatidylglycerol were placed in Put it in a 500ml round bottom flask, dissolve it with 150ml chloroform, evaporate the chloroform on a rotary evaporator on a constant temperature water bath at 55-60°C at 100rpm and reduce pressure, so that the composition forms a uniform lipid film at the bottom of the flask, Add 10ml of deionized water, sonicate for 10min, 0.45μm, 0.22μm, and filter the membrane to complete the particle, and the long-circulating liposome containing cabazitaxel is obtained.
Embodiment 3
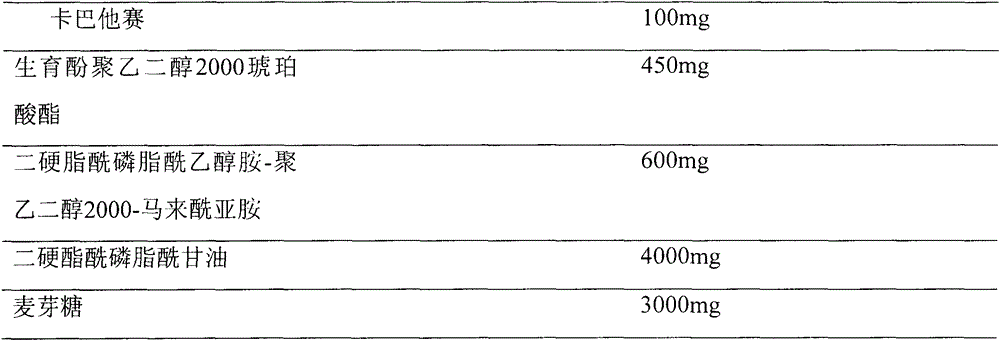
[0034] Prescription (100ml capacity)
[0035]
[0036] Preparation Process:
[0037] Prescribed amounts of cabazitaxel, tocopheryl macrogol 2000 succinate, distearoylphosphatidylethanolamine-polyethylene glycol 1200-maleimide and distearoylphosphatidylglycerol were placed in the In a 500ml round bottom flask, after dissolving with 150ml of acetone / methanol (1:1) solvent, evaporate the solvent on a rotary evaporator on a constant temperature water bath at 55 to 60°C at 100rpm and under reduced pressure, so that the composition forms a mixture at the bottom of the flask. Homogenize the lipid film, and add 6% maltose solution into the above-mentioned round bottom flask, and wash the film with a rotary evaporator at 50°C until the lipid film is hydrated and becomes a white liposome suspension. 0.22 μm, membrane sizing (3 times each), the final dispersion was divided into vials, and then freeze-dried to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com