Epoxide hydrolase as well as encoding gene and application thereof
A technology of epoxides and coding genes, which is applied in the field of enzyme catalysis and can solve problems such as inability to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
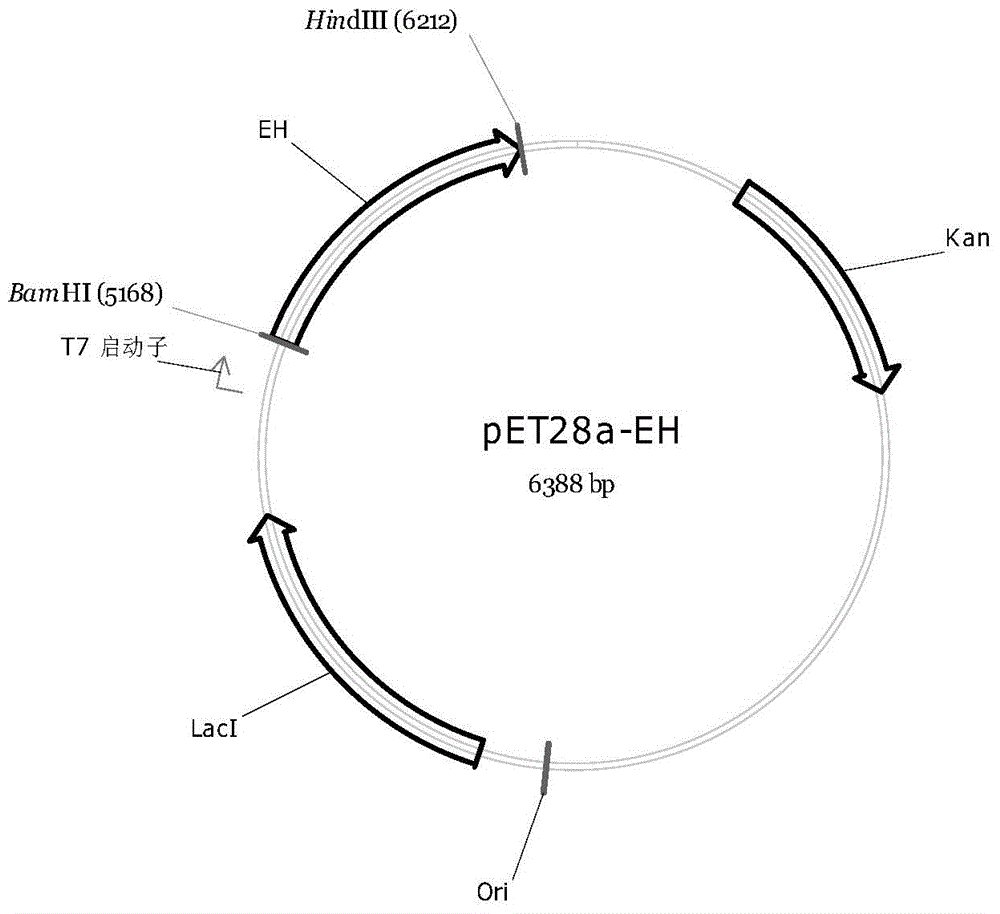
[0023] Example 1: Cloning containing epoxy hydrolase gene
[0024] Purchase a standard strain freeze-dried tube numbered DSM20162 from DSMZ, Germany.
[0025] 1LTsukamurella paurometabola medium is as follows: Trypticase Soy Broth (BBL11768, Oxoid CM129 or Merck 5459) 30.0g; distilled water 1000ml; PH7.3. Sterilize at 121°C for 15min. Incubate at 28°C in a constant temperature shaker at 200 rpm for 1 to 3 days, collect the bacteria by centrifugation, and extract the Tsukamurella paurometabola genome according to the instructions of the Gerui Bacterial Genome Extraction Kit, and amplify it with the following two primers:
[0026] F: 5’-CGC GGATCC ATGACGATCACCCCGCACACCGTG-3' (SEQ ID NO: 3);
[0027] R: 5’-CCC AAGCTT TCAGCCCGCGGGGTGGTTCTCC-3' (SEQ ID NO: 4);
[0028] The full length of the gene of the epoxy hydrolase is obtained.
[0029] Toyobo KOD enzyme PCR reaction system is: H 2 O 32.5ul, buffer 5ul, 2mm dNTP5ul, MgCl 2 2.5ul, template 2ul, 20mM F / R primer 1ul, KOD enzyme 1ul. Aft...
Embodiment 2
[0030] Example 2: Obtaining whole cells containing epoxy hydrolase
[0031] The obtained transformants were inoculated into LB liquid medium containing 50μg / ml kanamycin resistance, cultured at 37°C for 12h, and then inoculated with 1% of the inoculum amount (v / v) to freshly containing 50ug / ml kanamycin In the LB liquid medium resistant to mycin, culture at 37°C until the cell concentration OD600 is about 0.6, and then add IPTG with a final concentration of 0.1mM to the LB liquid medium. After inducing and culturing at 20°C for 20 hours, the culture solution is incubated at 4 Centrifuge at 8000 rpm for 10 min, discard the supernatant, and collect the precipitate, which is the recombinant E. coli BL21 / pET28a-EH wet bacteria containing the intracellular expression recombinant plasmid. Freeze-dried cells were obtained after the wet cells were freeze-dried for 4 hours. The formula of 1000ml LB medium is: peptone 10g, sodium chloride 10g, yeast extract 5g. The wet bacteria are broke...
Embodiment 3
[0032] Example 3: Application of the epoxy hydrolase in the preparation of R-phenyl glycidyl ether
[0033] The freeze-dried bacterial cells obtained in Example 2 were used as a catalyst. Dissolve 50 mg of lyophilized bacteria in 45 mL of 100 mM Trish-Hcl, mix well at 200 rpm for 5 min, and add 5 ml of racemic phenyl glycidyl ether dissolved in 4M DMSO at 200 rpm at 30° C. and terminate the reaction after 1 hour. Use 15ml n-hexane to extract three times to get ee> 99% R-phenyl glycidyl ether, then 15ml ethyl acetate extraction three times to get ee> 83% S-phenoxy glycol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com