A kind of porcine antimicrobial peptide cecropin P1 mutant and its preparation method and application
An antibacterial peptide and mutant technology, applied in the field of functional peptide screening, can solve the problems of unclear systemic or local toxicity, instability and drug resistance, and achieve broad market prospects and development value, antibacterial spectrum. The effect of broad and high antibacterial titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Acquisition of novel porcine-derived antimicrobial peptide cecropin P1 mutant and its gene
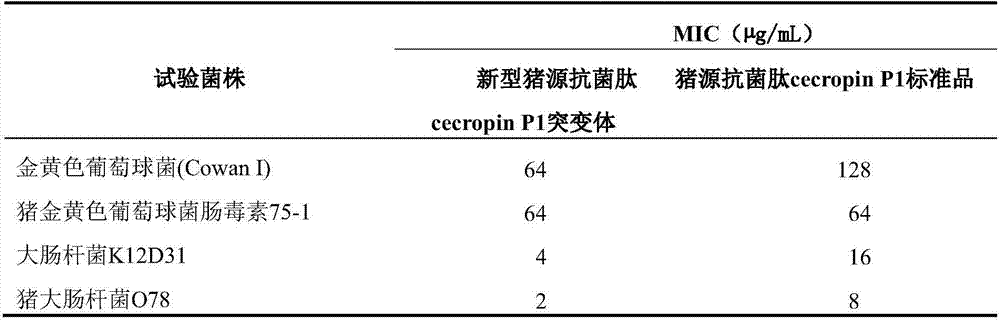
[0015] 1. Porcine antibacterial peptide cecropin P1 (GenBank accession number: AB186032) is an antibacterial peptide with amphipathic α-helix composed of 31 amino acids, which has strong antibacterial activity against bacteria. In order to further improve its antibacterial activity, the spatial structure analysis of the amino acid sequence of the porcine antimicrobial peptide AB186032 was carried out by biological software, and the two amino acids (10L, 12N) of the alpha helix at the hydrophilic end were selected to use the more hydrophilic arginine Acid (R) and lysine (K) were replaced in order to further improve the hydrophilicity and its ability to bind to the cell membrane, while adding asparagine at the C-terminus of the antimicrobial peptide to promote the amidation of the C-terminus, and finally A novel porcine antimicrobial peptide cecropin P1 mutant is obtaine...
Embodiment 2
[0017] Example 2 Construction of genetically engineered pig-derived antimicrobial peptide cecropin P1 mutant expression vector and acquisition of engineering bacteria
[0018] 1. Both the vector containing the antimicrobial peptide gene and the yeast expression vector were digested with XhoI and XbaI, and the digested products were recovered and ligated for PCR identification and sequencing.
[0019] 2. After the positive plasmid was linearized by SacI single enzyme digestion, it was added to the competent cell suspension of Pichia pastoris. After electroporation, spread evenly on YPDS selection plate containing 100 μg / mL Zeocin, and incubate at 30°C for 3-5 days. When the positive transformants on the YPDS plate grow larger, each transformant is inoculated onto the YPDS selection plate containing Zeocin 200 μg / mL, 500 μg / mL, and 1000 μg / mL in turn, and the colonies that grow normally on the high-concentration Zeocin plate are Possibly high copy recombinant strains.
[0020]...
Embodiment 3
[0021] Example 3 Fermentation and purification of novel porcine-derived antimicrobial peptide cecropin P1 mutant preparation
[0022] 1. Fermentation process
[0023] 1) Inoculate the positive recombinants obtained by screening into Erlenmeyer flasks with 1%-10% inoculum volume after activation, and inoculate 10L with 5%-20% inoculation volume at 28-30°C and 200r / min shaker for 16-24h Fermentation tank (installed culture medium 6L), temperature 28-30 ℃, rotation speed 500-1500r / min, medium pH value 5.0-6.0, ventilation volume 0.1-1.0VVM (the amount of oxygen introduced into 1L fermentation broth for 1min), dissolved Fermentation is carried out under the condition of oxygen>20%, and 50% glycerol is fed for 4 hours after culturing for 18-24 hours. When the dissolved oxygen suddenly rises to 100%, methanol is fed until the end of fermentation. The whole fermentation lasts for 48-72 hours.
[0024] 2) After the fermentation, the original tank was steam sterilized at 100°C for 10-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com