Functionalized icariin derivatives, and preparation method and application thereof
An icariin, functionalized technology, applied in the field of biomedical materials, can solve problems such as limited loading, and achieve the effects of slowing the release rate, low cytotoxicity, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
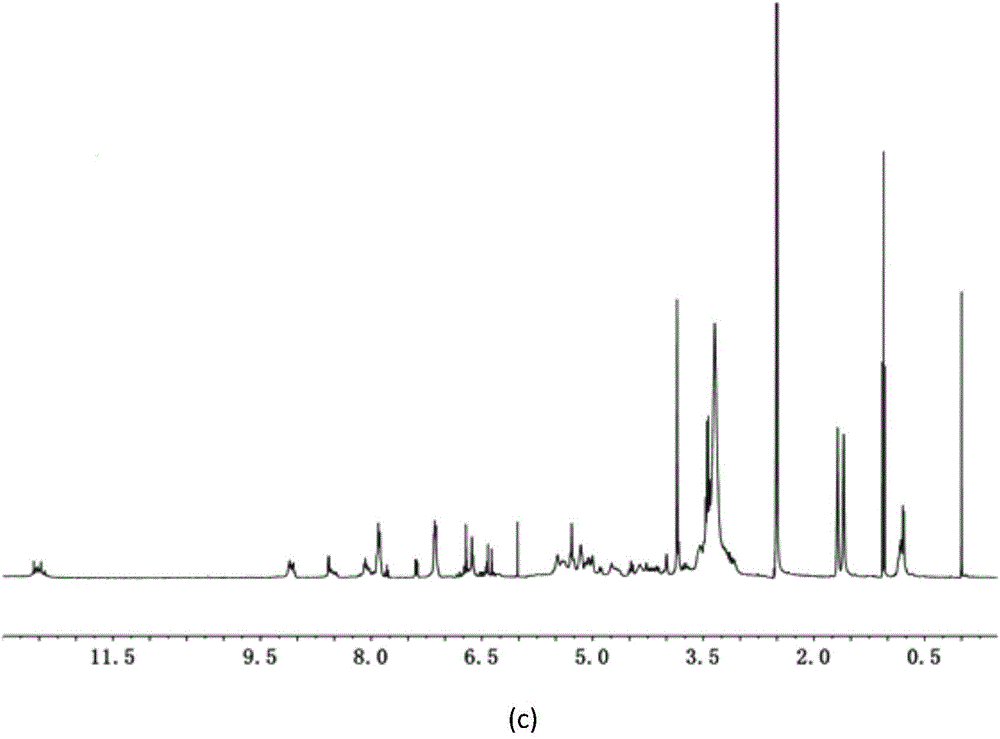
Image
Examples
Embodiment 1
[0046] Using icariin and succinic anhydride as raw materials, the molar ratio of icariin and succinic anhydride is 1:10.8.
[0047] Weigh 200mg of icariin and dissolve it in 2ml of pyridine, weigh 320mg of succinic anhydride and dissolve it in 2ml of pyridine, add the succinic anhydride solution to the icariin solution dropwise under stirring, mix well, and react at 25°C After 12 hours, most of the solvent was distilled off under reduced pressure in a rotary evaporator after the reaction was over to obtain a light yellow paste solid, which was dissolved in 20 mL of organic extractant ethyl acetate, then added 50 mL of deionized water, at 60 Stir and wash at ℃ for 15 minutes, let stand to separate the layers, separate the water layer with a separatory funnel, then add 50ml deionized water to the ethyl acetate layer containing the derivative to wash, repeat the operation several times until the pH value of the water layer tends to neutral. Then the ethyl acetate layer was trans...
Embodiment 2
[0050] Weigh 200mg of icariin and dissolve it in 2ml of pyridine, weigh 320mg of succinic anhydride and dissolve it in 2ml of pyridine, add the succinic anhydride solution to the icariin solution dropwise under stirring, mix well, and react at 25°C After 4 hours, most of the solvent was removed by distillation under reduced pressure in a rotary evaporator after the reaction was over to obtain a light yellow paste solid, which was dissolved with 20 mL of organic extractant ethyl acetate, then added 50 mL of deionized water, at 60 Stir and wash at ℃ for 15 minutes, let stand to separate the layers, separate the water layer with a separatory funnel, then add 50ml deionized water to the ethyl acetate layer containing the derivative to wash, repeat the operation several times until the pH value of the water layer tends to neutral. Then the ethyl acetate layer was transferred, and the ethyl acetate was volatilized in an ultra-clean bench, and then freeze-dried with a freeze dryer fo...
Embodiment 3
[0052] Using icariin and succinic anhydride as raw materials, the molar ratio of icariin and succinic anhydride is 1:10.8.
[0053] Weigh 200mg of icariin and dissolve it in 2ml of pyridine, weigh 320mg of succinic anhydride and dissolve it in 2ml of pyridine, add the succinic anhydride solution dropwise into the icariin solution and mix evenly under stirring, and stir at 40°C React for 4 hours. After the reaction was completed, most of the solvent was evaporated in an ultra-clean bench window to obtain a light yellow paste solid. Dissolve the obtained paste solid with 20mL of organic extractant ethyl acetate, then add 50ml of deionized water, stir and wash at 60°C for 15min, after standing for layers, separate the water phase with a separatory funnel, and then pour it into the derivative containing 50 ml of deionized water was added to the ethyl acetate layer for washing, and the operation was repeated several times until the pH value of the aqueous phase tended to be neutra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com