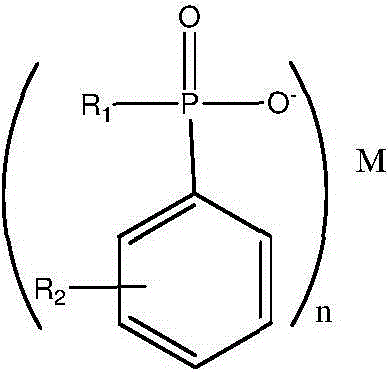
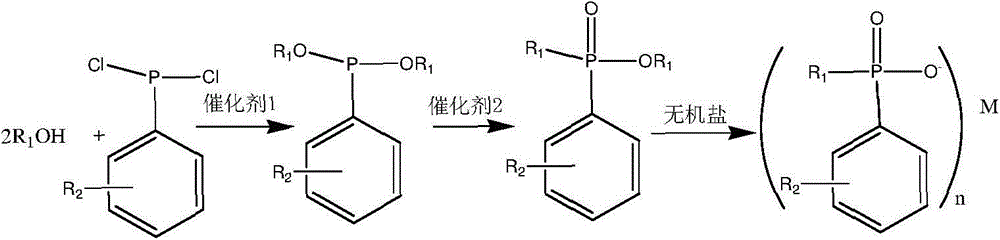
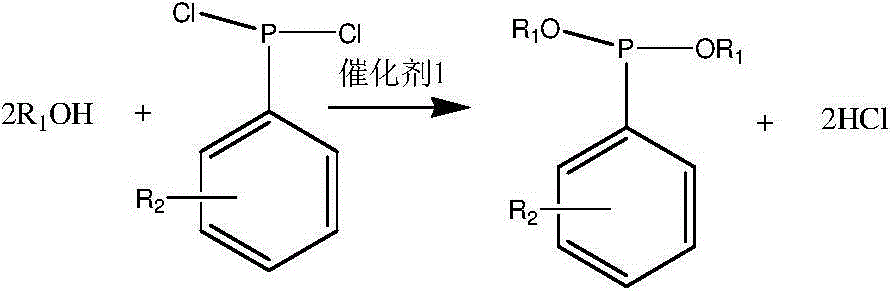
Synthesis process of phosphinate containing alkylaryl
A technology of alkylaryl phosphinate and alkylaryl phosphonate, which is applied in the field of synthesis of organic phosphinate flame retardants, can solve problems such as reducing reaction efficiency, and achieve abundant raw material sources and excellent reaction conditions Gentle, easy-to-use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 aluminum ethyl phenyl phosphinate
[0038] The first step: the synthesis of diethyl phenylphosphonite
[0039] Add 27.6g of ethanol, 22.2g of triethylamine and 100ml of chloroform into a flask equipped with a stirring, condensing, and drying device, start stirring, cool to 0°C in an ice-water bath, and slowly add 35.9 The chloroform solution of g phenylphosphorus dichloride was dropped in about 2 hours; keep at 0-10°C, monitor the reaction by PNMR, and stop the reaction after about 3 hours. After the reaction was completed, the generated solid was filtered off, the solvent and excess ethanol were removed by spin (steaming), and the obtained product was purified by distillation under reduced pressure, and a fraction at 107-109°C / 12mmHg was cut off. By weighing, 32.5 g of the product was obtained, and the product yield was 82.1%.
[0040] The second step: the synthesis of phenyl ethyl phosphonate
[0041] Put 32.3 g of diethyl phenylphosph...
Embodiment 2
[0044] The synthesis of embodiment 2 butylphenylphosphinate aluminum
[0045] The first step: the synthesis of dibutyl phenylphosphonite
[0046] In a four-neck flask equipped with stirring, condensation, and drying devices, add 32.4 g of butanol, 20.2 g of triethylamine, and 110 ml of 1,2-dichloroethane, start stirring, and cool to 3 ° C in an ice-water bath. Slowly add 35.7g of phenylphosphorous dichloride dissolved in 1,2-dichloroethane solution dropwise into the funnel, and the drop is completed in about 2.5 hours; keep at 2-8°C, monitor the reaction by PNMR, and stop the reaction after about 3 hours. After the reaction was completed, the generated solid was filtered off, the solvent and excess butanol were spun off, and the obtained product was purified by distillation under reduced pressure, and the 121-124°C / 12mmHg fraction was intercepted. By weighing, 42.2 g of the product was obtained, and the product yield was 83.0%.
[0047] The second step: the synthesis of phen...
Embodiment 3
[0050] In a flask equipped with a stirring, condensing, and drying device, take 40.1 g of the phenylbutyl phosphonate obtained in step 2, add 130 ml of N, N-dimethylformamide, and 38.9 g of aluminum trichloride, and start stirring device, under the protection of nitrogen, the temperature was raised to 117°C, the chlorobutane generated in the reaction was collected, and the reaction was monitored by PNMR. The reaction was completed after 3 hours of reaction, cooled down, filtered, and the filter cake was washed with acetonitrile and water, dried, and pulverized to obtain butyl 30.6g of aluminum phenyl phosphinate, the total yield of product is 74.0%. The synthesis of embodiment 3 o-tolyl-methyl phosphinate zinc
[0051] The first step: the synthesis of dimethyl o-methylphenylphosphonite
[0052] In a four-necked flask equipped with a stirring, condensing, and drying device, add 19.0 g of methanol, 15.8 g of pyridine, and 110 ml of 1,2-dichloroethane, start stirring, cool to 3°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com