Perylene diimide derivative, and application thereof in solar cell and preparation method of perylene diimide derivative
A technology of perylene diimide and solar cells, which is applied to circuits, photovoltaic power generation, electrical components, etc., can solve the problems of low yield of perylene diimide derivatives, less derivative products, etc., so as to improve the yield and improve the variety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038]A preparation method of perylene diimide derivatives, comprising:
[0039] (1) Add compound 1,6,7,12-tetrabromo-cyclohexylperylenediimide, phenylboronic acid, cesium fluoride, and silver oxide into a three-necked flask, and under nitrogen atmosphere, the catalyst tetrakis(triphenyl Base phosphine) palladium joins in the above-mentioned system of there-necked flask;
[0040] (2) Add the solvent used to obtain the derivative into the three-necked flask, mix and heat the reaction for 20-26 hours;
[0041] (3) Cool to room temperature and separate with a silica gel column to obtain the obtained product.
[0042] An organic solar cell comprising a donor polymer and the above-mentioned perylene diimide derivative as an acceptor material.
[0043] A method for applying perylene diimide derivatives to solar cells, comprising:
[0044] (1) Prepare 1,6,7,12-tetrasubstituted perylene diimide and donor polymer material into 20-40 mg / mL dichlorobenzene ( or chlorobenzene) solutio...
Embodiment 1
[0051]
[0052] The preparation method of this perylene diimide derivative is:
[0053]
[0054] Specific steps include:
[0055] 1) compound 1,6,7,12-tetrabromo-cyclohexyl perylene diimide, (391mg, 0.45mmol), phenylboronic acid (330mg, 2.7mmol), cesium fluoride (550mg, 3.6mmol) and Silver oxide (460mg, 2.0mmol) was added into the three-neck flask. Under nitrogen atmosphere, the catalyst tetrakis(triphenylphosphine)palladium (52mg, 0.045mmol) was added to the above system. After 15 mL of solvent (ethylene glycol dimethyl ether) was added, the mixed system was heated and reacted for 24 hours. Cool to room temperature and separate with a silica gel column to obtain a purple-black solid with a yield of 32%.
[0056] 2) Product characterization data:
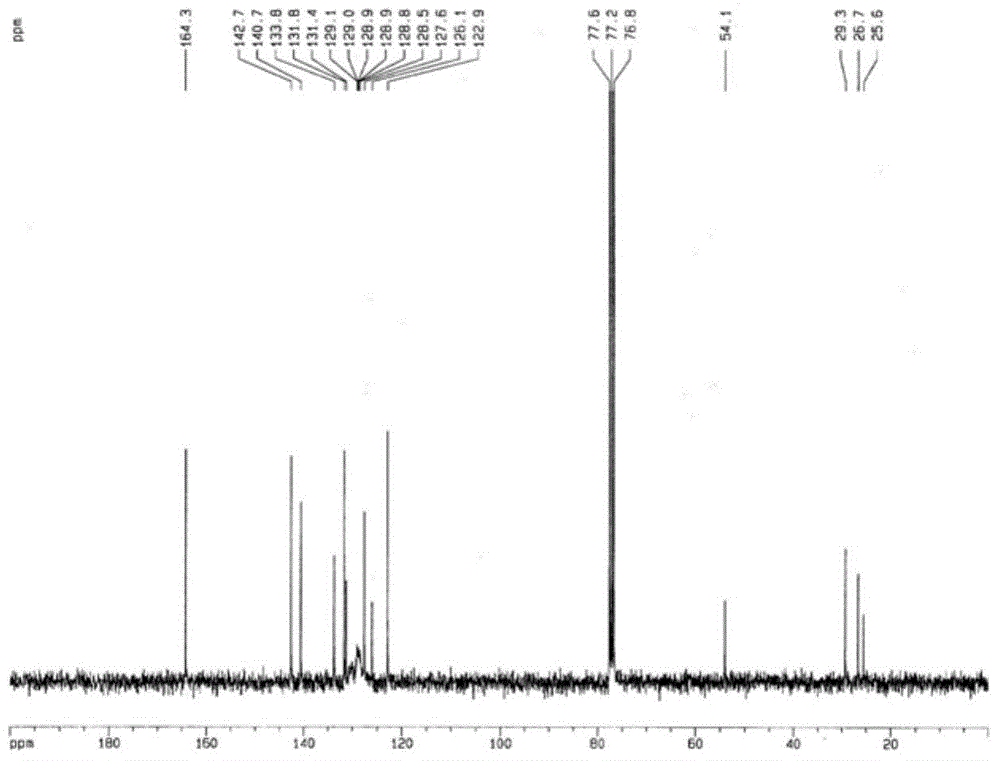
[0057] figure 1 It is the mass spectrum of the obtained perylene diimide derivative: calcd for C 60 h 46 N 2 o 4 859.02; found: 858.8.
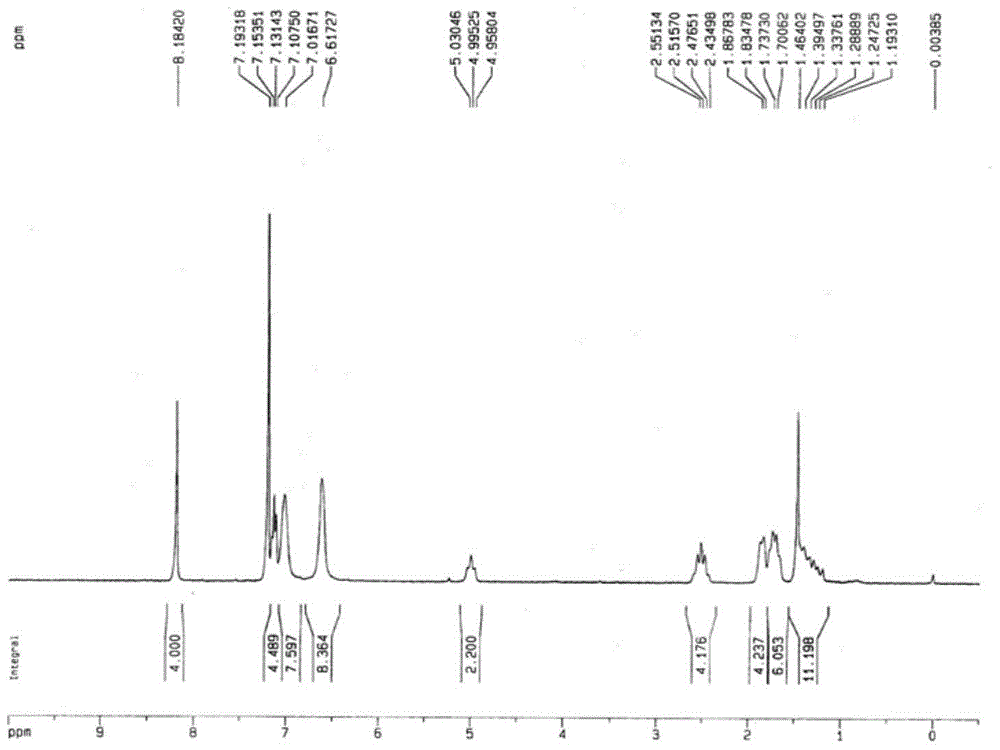
[0058] figure 2 The H NMR spectrum of the obtained perylene diimide derivativ...
Embodiment 2
[0062] The structural formula of the perylene diimide derivative of this embodiment is:
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com