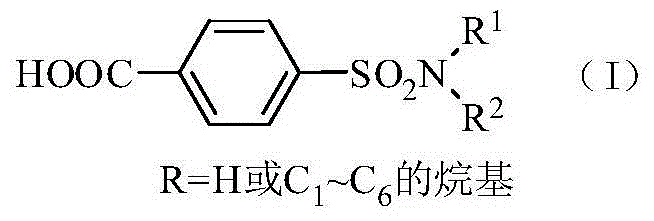
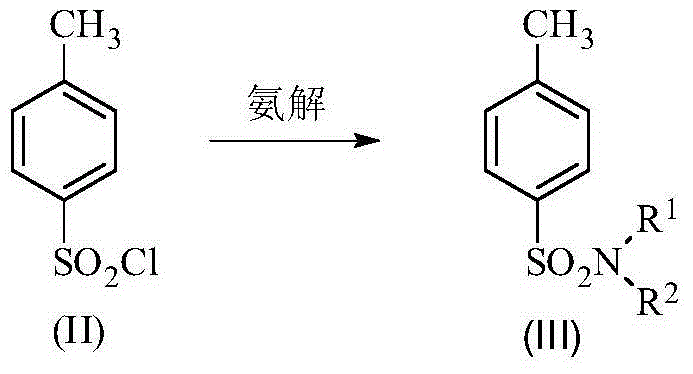
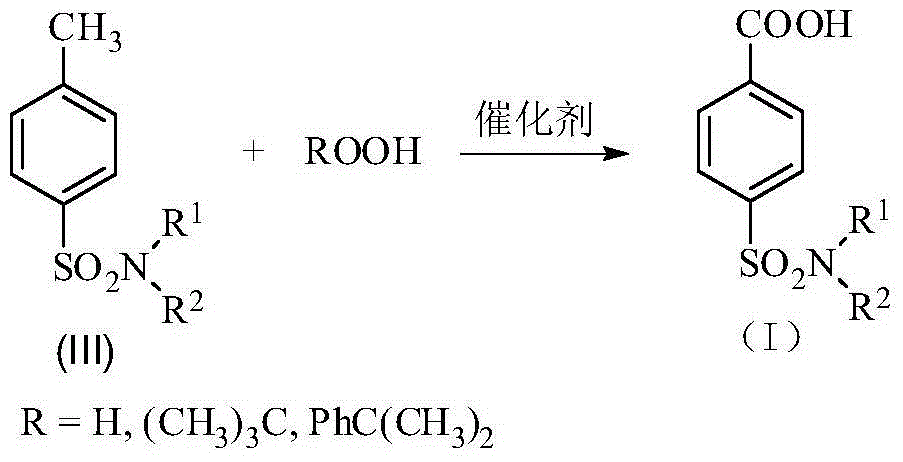
Method for synthesis of p-carboxybenzene sulfonamide through catalytic oxidation
A technology for carboxylbenzenesulfonamide and methylbenzenesulfonamide, which is applied in the field of catalytic oxidation synthesis of p-carboxybenzenesulfonamide, can solve the problems of inability to catalyze the oxidation of methylbenzenesulfonamide, equipment corrosion, complicated preparation, etc., and achieves stable properties, Cost saving, simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Step 1: In a 1L three-necked flask equipped with a condenser, a stirrer and a thermometer, add 380mL of ammonia water, then grind and pulverize 1mol p-toluenesulfonic acid chlorine, dissolve it in 300mL of toluene, and quickly add it to the three-necked flask , fully stirred, the reaction solution was milky, stirred at room temperature for 1-1.5 hours, cooled to 0°C, white crystals precipitated, filtered, the filter cake was washed with water, drained, weighed about 140g after drying, melting point 136-137 ℃. Yield 81.87%.
[0046] Step 2: Weigh 0.1mol of p-carboxybenzenesulfonamide, CeO 2 Add 0.005mol and 0.2mol of tert-butyl hydroperoxide into a 100mL reaction flask, adjust the pH to 12 with hydrochloric acid and sodium hydroxide, and then react at 80°C for 10h. After the reaction is complete, cool to room temperature. The catalyst is removed by filtration, washed and dried with water, and recycled. 6 mol / L HCl was added dropwise to the filtrate to adjus...
Embodiment 2
[0047] Embodiment 2: Step 2: Weigh 0.1mol of p-carboxybenzenesulfonamide, 0.002mol of TiO 2 , 0.8mol of tert-butyl hydroperoxide was added to the glass reactor, hydrochloric acid and sodium hydroxide were used to adjust the pH to 10, and then reacted at 70°C for 8h. After the reaction was completed, it was cooled to room temperature. The catalyst is removed by filtration, washed and dried with water, and recycled. 6 mol / L HCl was added dropwise to the filtrate to adjust the pH=2 to 3, and white crystals of p-carboxybenzenesulfonamide were precipitated. Filtrate, wash until neutral, and dry to obtain p-carboxybenzenesulfonamide with a yield of 81.83%. The purity of the product is 92.54% after analysis by Agilent 1200 high performance liquid chromatography.
[0048] Others are the same as in Example 1.
Embodiment 3
[0049] Embodiment 3: Step 2: Weigh 0.1mol of p-carboxybenzenesulfonamide, 0.001mol of WO 3 , 0.6mol of tert-butyl hydroperoxide was added to a 100mL reaction flask, hydrochloric acid and sodium hydroxide were used to adjust the pH to 14, and then reacted at 100°C for 1 hour. After the reaction was completed, it was cooled to room temperature. The catalyst is removed by filtration, washed and dried with water, and recycled. 6mol / L HCl was added dropwise to the filtrate to adjust the pH=3-4, and white crystals of p-carboxybenzenesulfonamide were precipitated. Filter, wash until neutral, and dry to obtain p-carboxybenzenesulfonamide with a yield of 82.85%. The purity of the product is 95.47% after analysis and detection by an Agilent 1200 type high performance liquid chromatograph.
[0050] Others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com