Preparation of dual-hepatic-targeting long-circulation gypenoside liposome and preparation method of liposome
A long-circulation liposome and liver-targeting technology, which is applied in liposome delivery, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the double liver-targeting long-circulation gypenoside liposome report that has not been seen and other issues, to achieve the effects of prolonging the circulation time in the body, enhancing stability, and enhancing targeting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
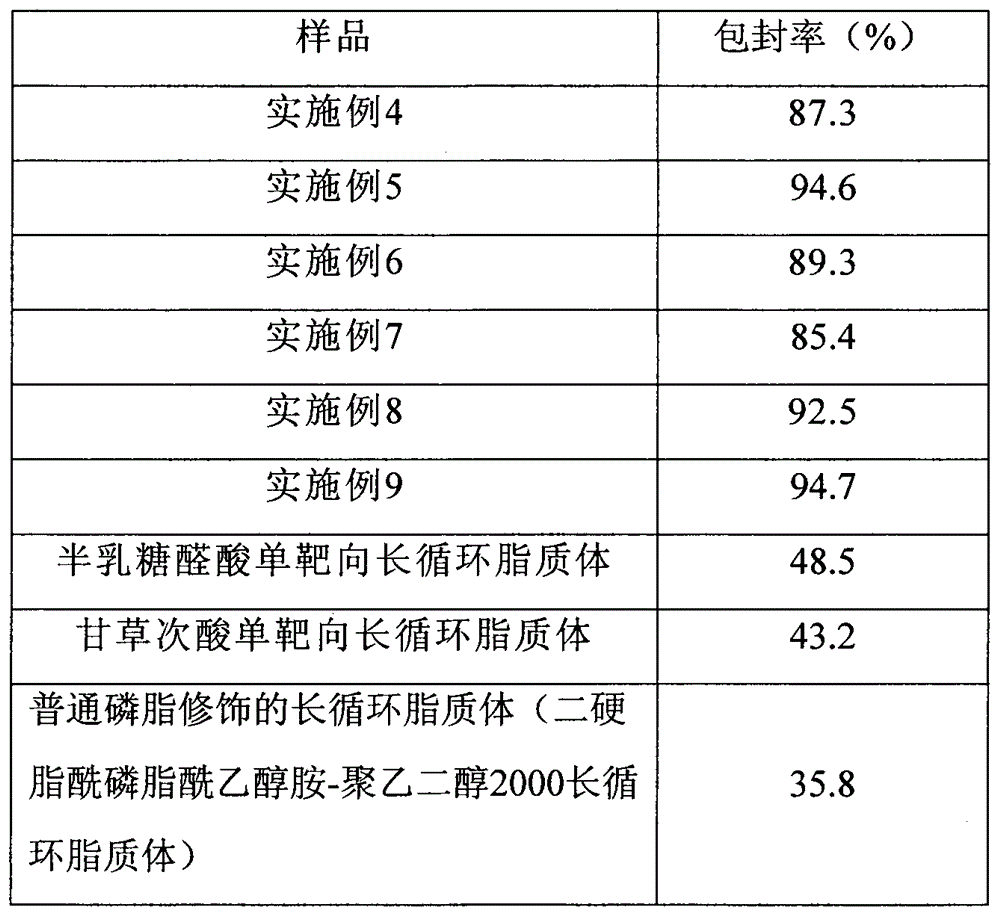
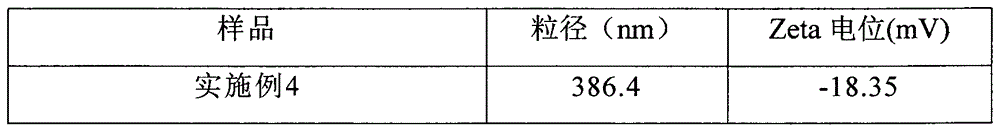
Examples
Embodiment 1
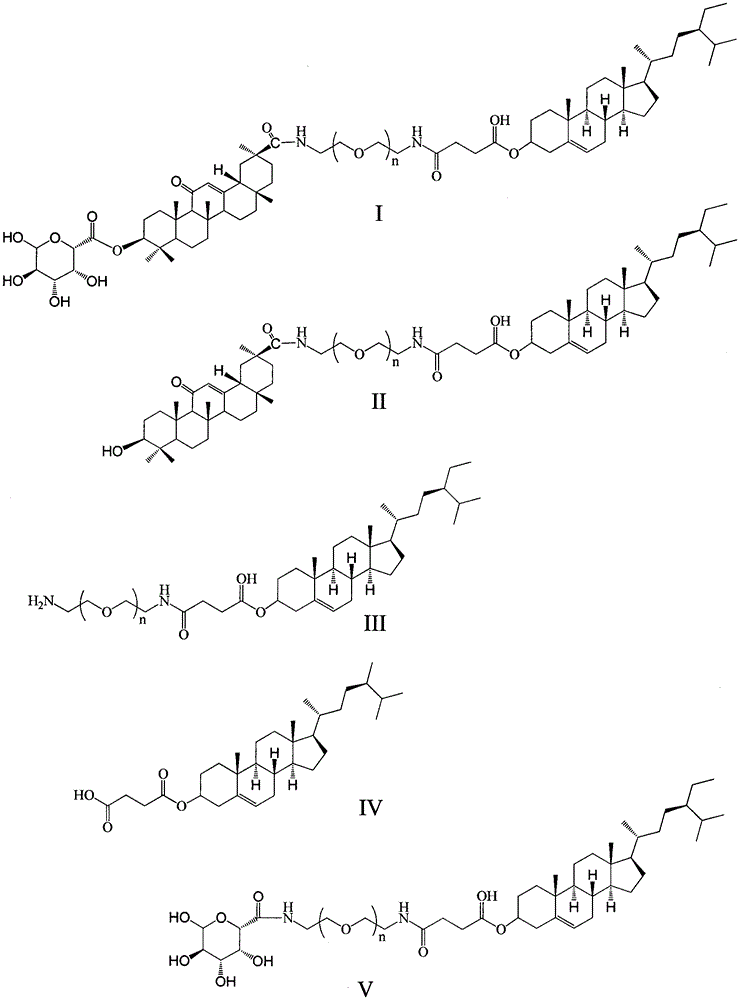
[0055] (1) (4.15g, 0.01mol) β-sitosterol, (0.80g, 0.008mol) succinic anhydride, (0.98g, 0.008mol) 4-dimethylaminopyridine are mixed in 100ml dichloromethane, in 45 ° C reaction, stirring for 48h. The dichloromethane solution was washed with 1mol / L (3×100mL) hydrochloric acid solution, Na 2 SO 4 Dry, filter and spin dry. Obtain the white solid compound shown in formula IV;
[0056] (2) (5.14g, 0.01mol) compound of formula IV, (16g, 0.008mol) double-ended amino polyethylene glycol 2000, (0.98g, 0.008mol) 4-dimethylaminopyridine, (1.53g, 0.008mol) ) 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was mixed in dichloromethane, reacted at 45°C, and stirred for 48h. The dichloromethane solution was washed with 1mol / L (3×100mL) hydrochloric acid solution, Na 2 SO 4 Dry, filter and spin dry. The crude product was subjected to flash column chromatography on silica gel (300-400 mesh), eluting with dichloromethane-methanol (100:1), and the collected solution was concent...
Embodiment 2
[0060] (1) Mix (4.15g, 0.01mol) β-sitosterol, (1.0g, 0.0125mol) succinic anhydride, (2.44g, 2mol) 4-dimethylaminopyridine in 100ml dichloromethane, at 45°C The reaction was stirred for 48h. The dichloromethane solution was washed with 1mol / L (3×100mL) hydrochloric acid solution, Na 2 SO 4 Dry, filter and spin dry. Obtain the white solid compound shown in formula IV;
[0061] (2) (5.14g, 0.01mol) compound of formula IV, (60g, 0.03mol) double-ended amino polyethylene glycol 2000, (3.67g, 0.03mol) 4-dimethylaminopyridine, (5.75g, 0.03mol) ) 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was mixed in dichloromethane, reacted at 45°C, and stirred for 48h. The dichloromethane solution was washed with 1mol / L (3×100mL) hydrochloric acid solution, Na 2 SO 4 Dry, filter and spin dry. The crude product was subjected to flash column chromatography on silica gel (300-400 mesh), eluting with dichloromethane-methanol (100:1), and the collected solution was concentrated und...
Embodiment 3
[0065] (1) (4.15g, 0.01mol) β-sitosterol, (5.0g, 0.05mol) succinic anhydride, (3.05g, 0.025mol) 4-dimethylaminopyridine are mixed in 100ml dichloromethane, at 45 ° C reaction, stirring for 48h. The dichloromethane solution was washed with 1mol / L (3×100mL) hydrochloric acid solution, Na 2 SO 4 Dry, filter and spin dry. Obtain the white solid compound shown in formula IV;
[0066] (2) (5.14g, 0.01mol) compound of formula IV, (30g, 0.015mol) double-ended amino polyethylene glycol 2000, (1.83g, 0.015mol) 4-dimethylaminopyridine, (2.88g, 0.015mol) ) 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride was mixed in dichloromethane, reacted at 45°C, and stirred for 48h. The dichloromethane solution was washed with 1mol / L (3×100mL) hydrochloric acid solution, Na 2 SO 4 Dry, filter and spin dry. The crude product was subjected to flash column chromatography on silica gel (300-400 mesh), eluting with dichloromethane-methanol (100:1), and the collected solution was concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com