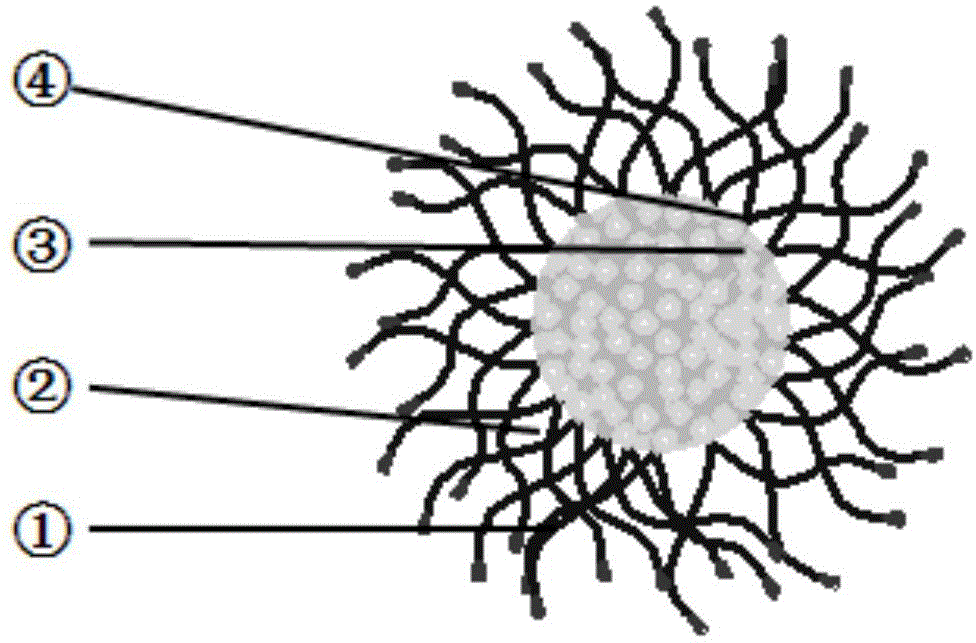
Polypyrrolidone polymer drug carrier micelle
A polypyrrolidone and polymer technology, which is applied in nano-drugs, microcapsules, drug combinations, etc., can solve the problems of low water solubility of drug molecules, high synthesis cost, poor biocompatibility, etc. Large volume and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] ① Preparation of azide small molecule initiator
[0045]Step 1: Dissolve 5 mL of 2-chloroethoxyethanol in 25 mL of butanone, and add 4.5 g of NaN to the solution 3 , 2.5gBu 4 NI, 10 mg of dicyclohexane-18-crown-6, the mixture was heated to boiling and stirred for 24 hours. The mixture was filtered and the precipitate was rinsed thoroughly with acetone. The resulting mixed solution is the crude product of the product, after the mixed solution was concentrated, at 90 o C was distilled to obtain the pure product. The resulting 2-azidoethoxyethanol 1 H NMR (CDCl 3 ): 3.70 (t, 2 H, C H 2 OH), 3.65 (t, 2 H, HOCH 2 C H 2 O), 3.56 (t, 2H, N 3 CH 2 C H 2 O), 3.37 (t, 2H, C H 2 N 3 ), and 2.56 (s, 1H, OH).
[0046] Step 2: Dissolve 2g of 2-azidoethoxyethanol and 5.09g of α-chloroacyl chloride in 30mL of dichloromethane prepared in step 1, transfer the reaction system to ice, and dissolve 6.8g of dicyclohexyl The carbodiimide was slowly added to the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com