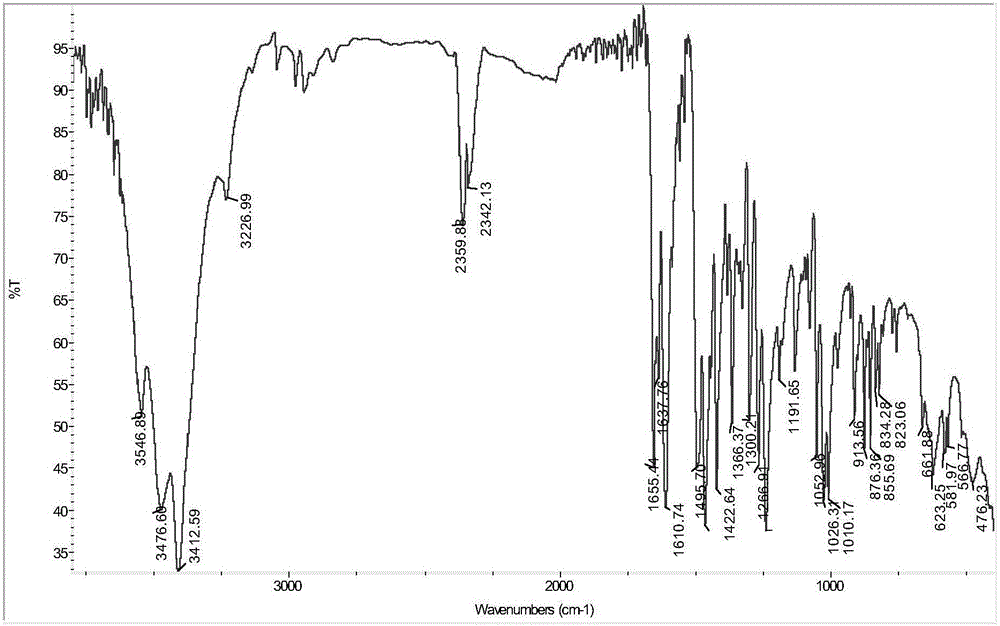
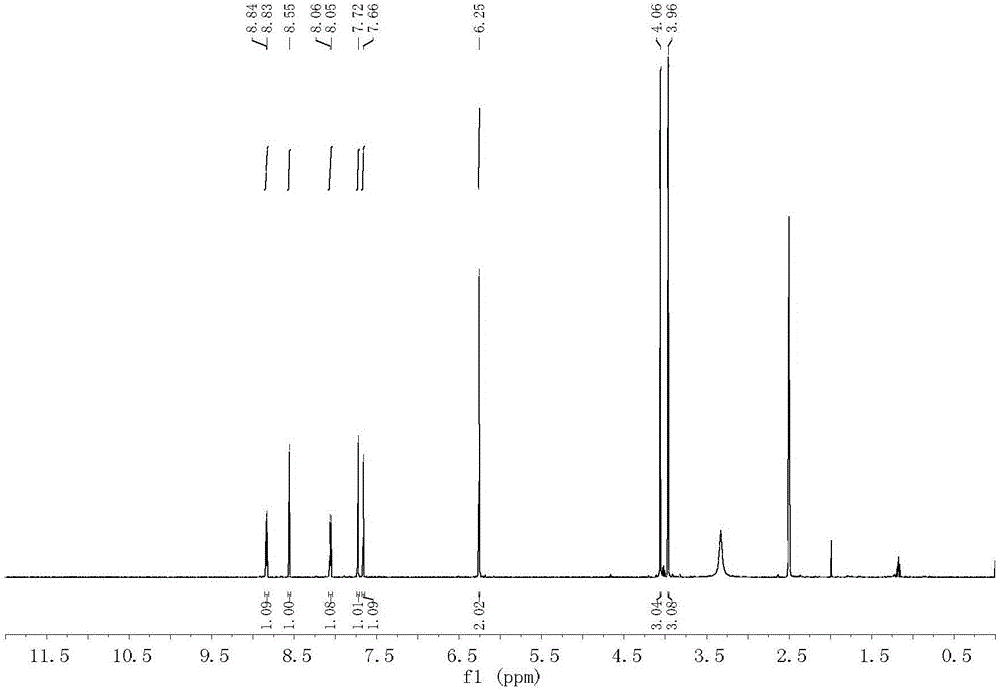
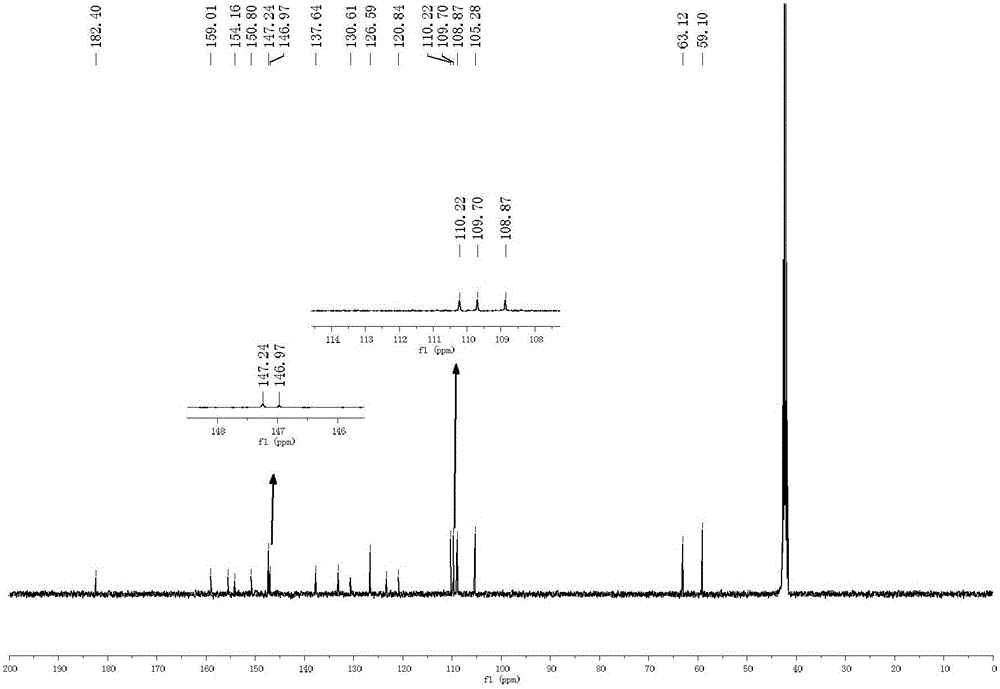
Synthesis method and application of oxidized nandinaphylline
A technology of nandinaphylline and its synthesis method, which is applied in the field of medicine and can solve problems such as the organic synthesis method of oxidized nandinaphylline that has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of oxidized nandinaphylline
[0050] 1) Synthesis of Intermediate 1:
[0051] Weigh 0.2 mol of pepper acetic acid, dissolve it in 200 mL of glacial acetic acid with stirring, and slowly add 0.2 mol of bromine water dropwise. After the dropwise addition, react at 10° C. for 1 h, then pour the reaction solution into ice water, let stand, and filter with suction to obtain a white powder of Intermediate 1 with a yield of 60%.
[0052] 2) Synthesis of Intermediate 2:
[0053] Weigh 0.2 mol of intermediate 1, dissolve it in 200 mL of chloroform, slowly add 0.3 mol of thionyl chloride, and react under reflux at 60° C. for 4 h. After cooling, the solvent was evaporated to dryness to obtain a brown oil, which was dissolved in 400 mL of chloroform to obtain solution A. Dissolve 0.2 mol of 3,4-dimethoxyphenethylamine in 400 mL of chloroform, then add 400 mL of ammonia solution, and stir to obtain solution B (at this time, the pH of solution B=8.5). Un...
Embodiment 2
[0073] 1) Synthesis of intermediate 1:
[0074] Weigh 0.2 mol of pepper acetic acid, dissolve it in 500 mL of glacial acetic acid with stirring, and slowly add 0.6 mol of bromine water dropwise. After the dropwise addition, the reaction was continued at 35° C. for 6 h, and then the reaction solution was poured into ice water, left to stand, and suction filtered to obtain a white powder of Intermediate 1 with a yield of 90%.
[0075] 2) Synthesis of Intermediate 2:
[0076] Weigh 0.2 mol of intermediate 1, dissolve it in a total of 600 mL of a solvent obtained by mixing dichloromethane and ethyl acetate at a ratio of 3:1, slowly add 0.8 mol of thionyl chloride, and react under reflux at 120°C for 12 hours. After cooling, the solvent was evaporated to dryness to obtain a brown oil, which was dissolved in 600 mL of dichloromethane to obtain solution A. Dissolve 0.2mol of 3,4-dimethoxyphenethylamine in 600mL of dichloromethane, then add 600mL of saturated aqueous sodium bicarbon...
Embodiment 3
[0088] 1) Synthesis of intermediate 1:
[0089] Weigh 0.2 mol of pepper acetic acid, dissolve it in 300 mL of glacial acetic acid with stirring, and slowly add 0.4 mol of bromine water dropwise. After the dropwise addition, the reaction was continued at 25° C. for 4 h, and then the reaction solution was poured into ice water, left to stand, and suction filtered to obtain a white powder of Intermediate 1 with a yield of 85%.
[0090] 2) Synthesis of Intermediate 2:
[0091] Weigh 0.2 mol of intermediate 1, dissolve it in 400 mL of ethyl acetate, slowly add 0.3-0.8 mol of thionyl chloride, and react under reflux at 100°C for 8 hours. After cooling, the solvent was evaporated to dryness to obtain a brown oil, which was dissolved in 500 mL of ethyl acetate to obtain solution A. Dissolve 0.2 mol of 3,4-dimethoxyphenethylamine in 500 mL of ethyl acetate, then add 500 mL of aqueous sodium hydroxide solution, and stir evenly to obtain solution B (at this time, the pH of solution B=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com