Thymine nucleobase-based triazolopyrimidines and production method therefor
A manufacturing method and a technology for triazololation, which can be used in the field of antitumor agents and can solve problems such as strong side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
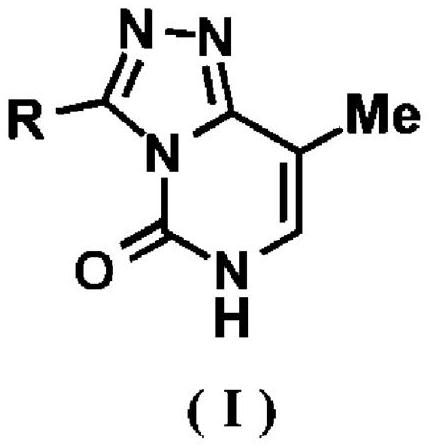
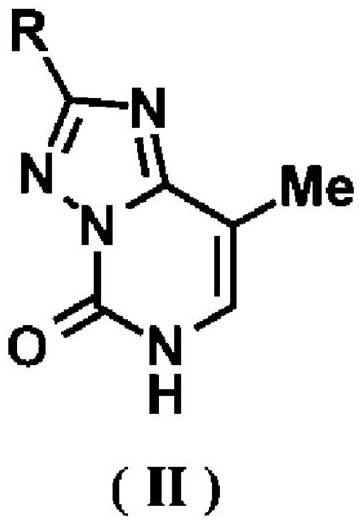
[0166] [Example 1.3-substituted 8-methyl-[1,2,4]triazolo[4,3- c ]pyrimidine-5(6 H )-keto compound 4a-f, s, t (general formula I) and 2-substituted 8-methyl-[1,2,4]triazolo[1,5- c ]pyrimidine-5(6 H )-Kone compound 5a-t (general formula II) synthesis example]
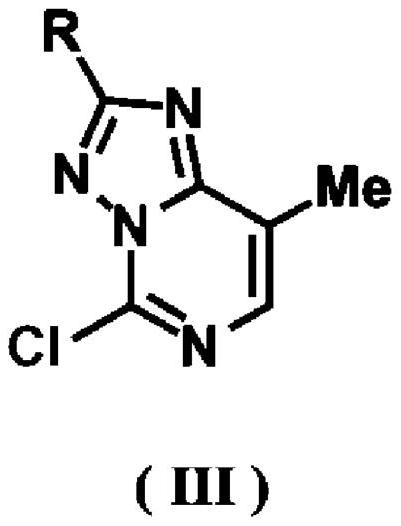
[0167] Triazolopyrimidine compounds represented by compounds 4a-f, s, t and 5a-t were synthesized according to the reaction formula described in Scheme 1 above.
Synthetic example 1
[0169] 4-hydrazino-5-methylpyrimidine-2(1 H )-Synthesis of ketone compound 2
[0170] Add 4-thiothymine compound 1 (1 g, 7.0 mmol) and hydrazine hydrate (2 g, 40 mmol) to EtOH (8 mL), and heat to reflux for 10 minutes. After the reaction, the precipitated crystals were collected by filtration. It was recrystallized from water to give colorless needles (0.80 g, 81%, mp>300°C).
[0171] [chemical formula 15]
[0172] 1 H NMR [200MHz, (CD 3 ) 2 SO]δ: 1.68 (3H, s, 5-Me), 5.82 (2H, br s, interchangeable with D20, NH 2 ), 6.68 (1H, br s, interchangeable with D20, 6-H), 9.41 (2H, br, interchangeable with D20, NH); IR: 3260 (ν as , NH 2 ), 3180(ν s , NH 2 ), 3130, 3060 (ν, NH), 1660 (ν, C=O), 1600cm -1 (δ, NH 2 ); analysis and calculation value C 5 h 8 N 4 O·1 / 2H 2 O: C, 40.26; H, 6.08; N, 37.56 Experimental value: C, 39.97; H, 5.96; N, 37.73; MS (FAB, glycerol matrix): m / z=141 (MH + ).
Synthetic example 2
[0174] 4-Alkylidenehydrazino-5-methylpyrimidine-2(1 H )-ketone and 4-arylmethylidenehydrazino-5-methylpyrimidine-2(1 H General Synthesis of )-Kone Compounds 3a-r
[0175] To MeOH (25 mL) was added 4-hydrazino-5-methylpyrimidine-2 (1 H )-ketone compound 2 (4mmol) and various aldehydes (4.8mmol), stirred at room temperature for 30 minutes to 2 hours. After the reaction, the precipitated crystals were collected by filtration and recrystallized from EtOH to obtain the corresponding target compounds 3a-r (Tables 1-4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com