New crystal forms of sodium 2-(5-bro-4-(4-cyclopropylnaphthalene-1- yl)-4H-1,2,4-triazole-3-yl sulfenyl) acetate and preparation method thereof
A technology of cyclopropylnaphthalene and thiol, which is applied in the field of chemical medicine, can solve the problems of mesophase crystallinity, mixed amorphous, crystal transformation, etc., achieve good stability, and is conducive to long-term storage and placement. Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Preparation of sodium 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate crystal form I:
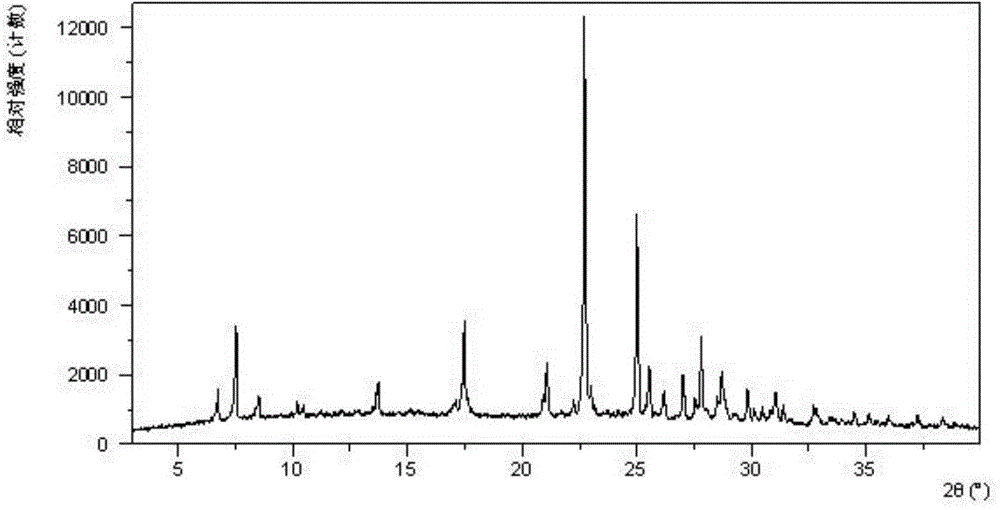
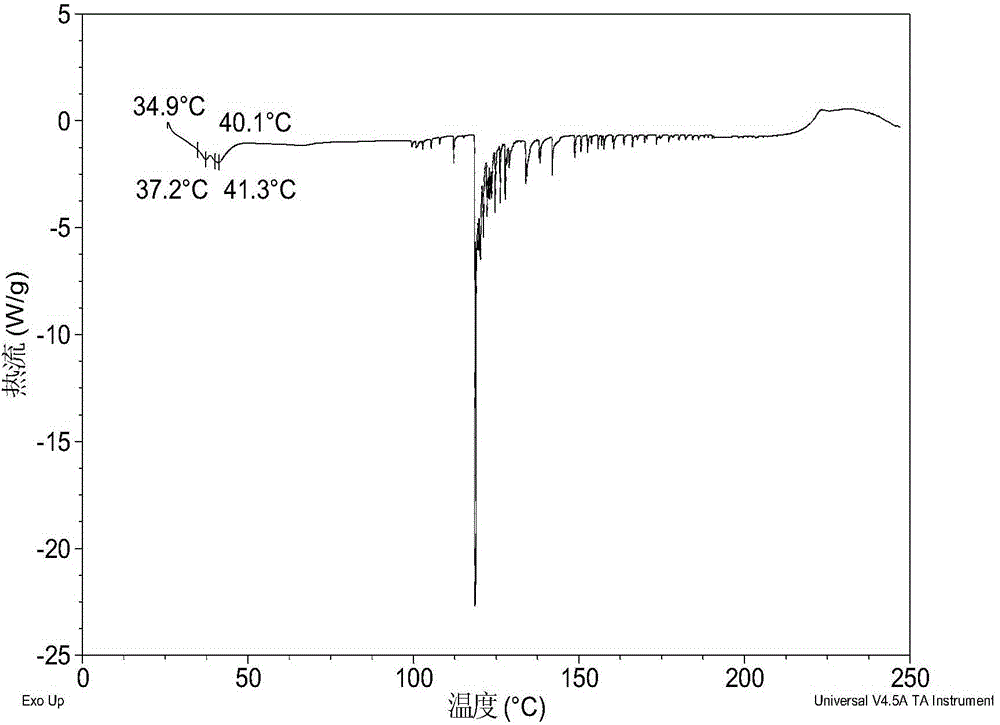
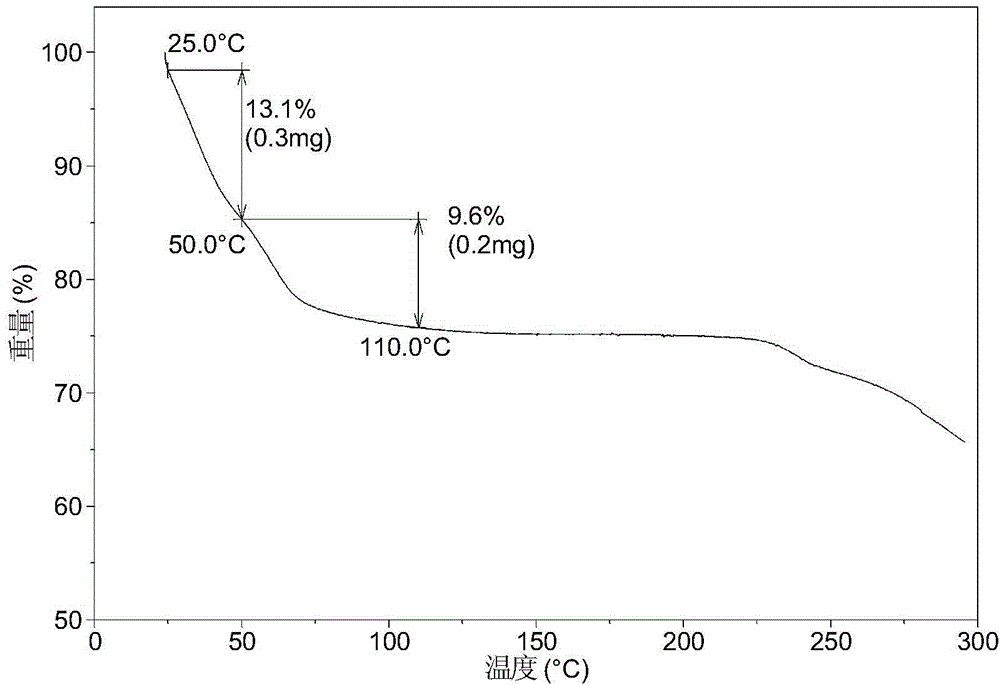
[0081] Dissolve 200 mg of amorphous sodium 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate in 1.0 mL In pure water, a clear solution was obtained, stirred at room temperature for 24 hours until solids were precipitated, and the wet solids were collected for XRPD testing to obtain Form I. Its XRPD pattern is as follows figure 1 As shown, the DSC diagram is shown in figure 2 As shown, the TGA graph is shown as image 3 shown.
[0082] Table 1 Powder X-ray Diffraction Data of Form I
[0083] 2theta
[0084] 17.55
[0085] 28.77
Embodiment 2
[0087] Preparation of sodium 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate crystal form II:
[0088] Dissolve 50 mg of amorphous sodium 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate in 0.5 mL In the mixed system of ethyl acetate: water = 976:24 (v:v), stir at room temperature for more than 48 hours, collect the solid to obtain the crystal form II. Its XRPD pattern is as follows Figure 5 As shown, the DSC diagram is shown in Image 6 As shown, the TGA graph is shown as Figure 7 shown.
[0089] Table 2 X-ray powder diffraction data of crystal form II
[0090] 2theta
[0091] 23.63
Embodiment 3
[0093] Preparation of sodium 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate crystal form III:
[0094] Dissolve 3.8 mg of amorphous sodium 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate in 2.0 mL of ethyl acetate solvent to obtain a clear solution, which was slowly evaporated at room temperature until solids were precipitated, and the solids were collected to obtain Form III. Its XRPD pattern is as follows Figure 9 As shown, the DSC diagram is shown in Figure 10 As shown, the TGA graph is shown as Figure 11 shown.
[0095] Table 3 X-ray powder diffraction data of crystal form III
[0096] 2theta
[0097] 20.05
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com