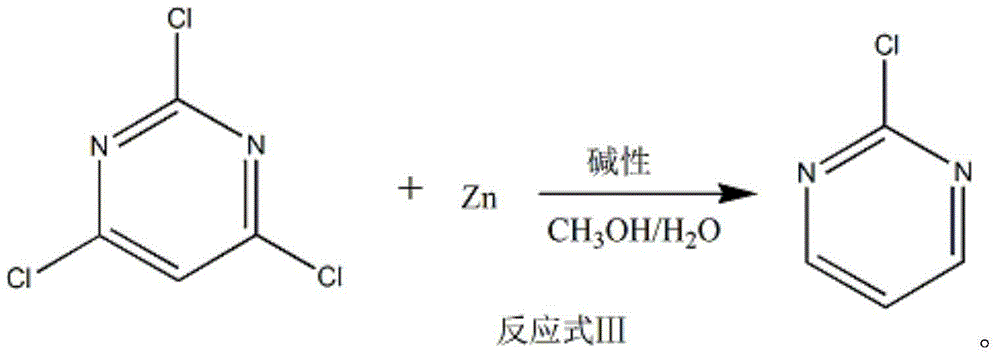
Method of preparing 2-chloropyrimidine
A chloropyrimidine and reaction technology, applied in the field of 2-chloropyrimidine preparation, can solve the problems of low product purity and low yield, and achieve the effects of high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
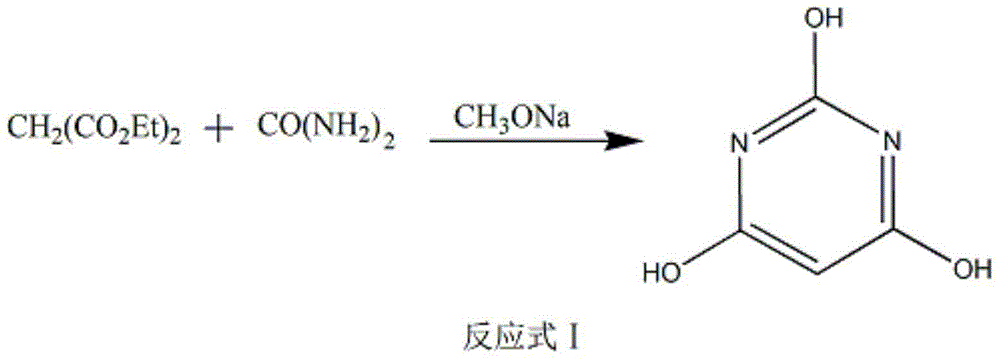
[0019] Put 1.05mol urea and 25wt% methanol solution of sodium methoxide (1.15mol in methanol) into a 1000mL four-neck bottle, slowly add 1mol diethyl malonate dropwise at reflux temperature, stir and reflux for 2 hours, and obtain a large amount of white powder The solid was concentrated under reduced pressure to obtain a white sodium salt dry powder, to which was added 38 wt% dilute hydrochloric acid solution, water was added to adjust the pH to 1.5, heated to 65°C, and allowed to react for 4 hours. After the reaction was completed, the temperature was lowered to 0° C. to precipitate crystals, and the crystals were filtered out. Recrystallize from a methanol / water mixed solution to obtain a white powder, which is the product barbituric acid.
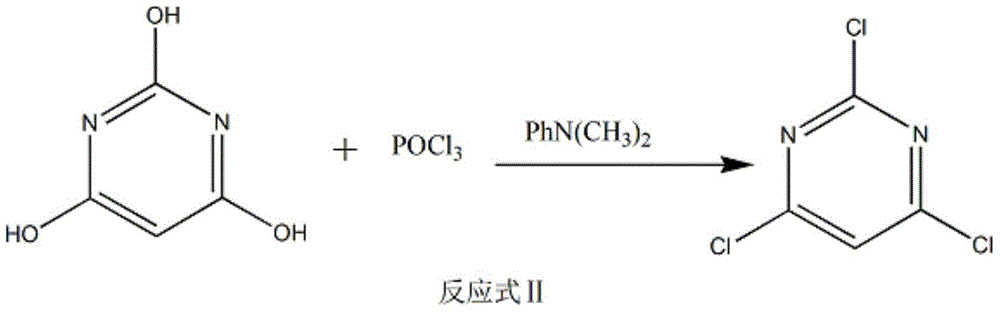
[0020] Put 5.1mol phosphorus oxychloride and N,N-dimethylaniline (60mL) into a 1000mL four-neck bottle, heat to 40°C, add barbituric acid in batches within 30-60min, and keep the temperature at 40°C . After adding the barbituric acid,...
Embodiment 2
[0023] Put 1.15mol of urea and 25wt% methanol solution of sodium methoxide into a 1000mL four-necked bottle (the methanol contains 1.25mol), slowly add 1mol of diethyl malonate dropwise at reflux temperature, stir and reflux for 3 hours, and obtain a large amount of white powder The solid was concentrated under reduced pressure to obtain a white sodium salt dry powder, to which was added 38wt% dilute hydrochloric acid solution, water was added to adjust the pH to 3, and heated to 75°C for 2 hours to react. After the reaction was completed, the temperature was lowered to 0° C. to precipitate crystals, and the crystals were filtered out. Recrystallize from a methanol / water mixed solution to obtain a white powder, which is the product barbituric acid.
[0024] Put 5.5mol phosphorus oxychloride and N,N-dimethylaniline (60mL) into a 1000mL four-necked bottle, heat to 40°C, add barbituric acid in batches within 30-60min, and keep the temperature at 50°C . After adding the barbitur...
Embodiment 3
[0027] Put 1.10mol of urea, 25wt% sodium methoxide (), methanol solution (1.20mol in methanol) into a 1000mL four-necked bottle, slowly add 1mol of diethyl malonate dropwise at reflux temperature, stir and reflux for 2 hours, and obtain a large amount of white The powdery solid was concentrated under reduced pressure to obtain a white sodium salt dry powder, to which was added 38 wt% dilute hydrochloric acid solution, water was added to adjust the pH to 2.5, heated to 65-75°C, and allowed to react for 3 hours. After the reaction was completed, the temperature was lowered to 0° C. to precipitate crystals, and the crystals were filtered out. Recrystallize from a methanol / water mixed solution to obtain a white powder, which is the product barbituric acid.
[0028] Put 5.3mol phosphorus oxychloride and N,N-dimethylaniline (60mL) into a 1000mL four-necked bottle, heat to 40°C, add barbituric acid in batches within 30-60min, and keep the temperature at 45°C . After adding the barb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com