Dual functionalized nanometer material, preparation method and uses thereof
A nanomaterial, bifunctional technology, applied in the field of preparation and application of bifunctional noble metal nanomaterials, can solve the problem that the luminous intensity cannot be improved to meet the requirements, etc., and achieve a good application prospect, good reproducibility and high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] B. Preparation method of bifunctional noble metal nanomaterials
[0081] The present invention also provides a method for preparing the bifunctional noble metal nanomaterial of the present invention easily and rapidly, which comprises the following steps:
[0082] 1. Provide coordination metal ion chelates containing mercapto or amino groups
[0083] The mercapto or amino-containing coordination metal ion chelate is as defined above, which comprises a coordination metal ion and a chelating agent, wherein the chelating agent can be obtained by performing a chelating agent that does not contain a mercapto or amino group known in the art. Thilated or aminated in the system. The thiolation or amination of such chelating agents is known, see for example: Christophe Alric, Jacqueline Taleb, Géraldine Le Duc, Céline Mandon, Claire Billotey, Alice Le Meur-Herland, Thierry Brochard, Francis Vocanson, Marc Janier, Pascal Perriat, Stéphane Roux, and Olivier Tillement. J. Am. Che...
Embodiment
[0117] testing method:
[0118] (1) Transmission electron microscope;
[0119] Test method: transmission electron microscope (TEM, JEOL Ltd, JEOL-2010, Japan). The prepared sample was centrifuged, and the supernatant was removed after 15 min at 12500*g. After the obtained precipitate was dispersed in absolute ethanol solution, transmission electron microscope analysis was performed.
[0120] (2) Thermogravimetric analysis and differential thermal analysis;
[0121] Test method: (TGA / DTG, Q5000V3.15) Centrifuge the prepared sample, remove the supernatant after 12500*g for 15min. The obtained precipitate was dried under vacuum condition, and thermogravimetric analysis and differential thermal analysis were carried out.
[0122] (3) Ultraviolet visible spectrum;
[0123] Test method: (Agilent8453 ultraviolet-visible spectrometer) centrifuge the prepared sample at 12500*g for 15 minutes and remove the supernatant. The resulting precipitate was dispersed in a pH=12 NaOH sol...
Synthetic example 1
[0128] Synthesis Example 1: Preparation of thiolated diethylenetriaminepentaacetic acid (chelating agent 1)
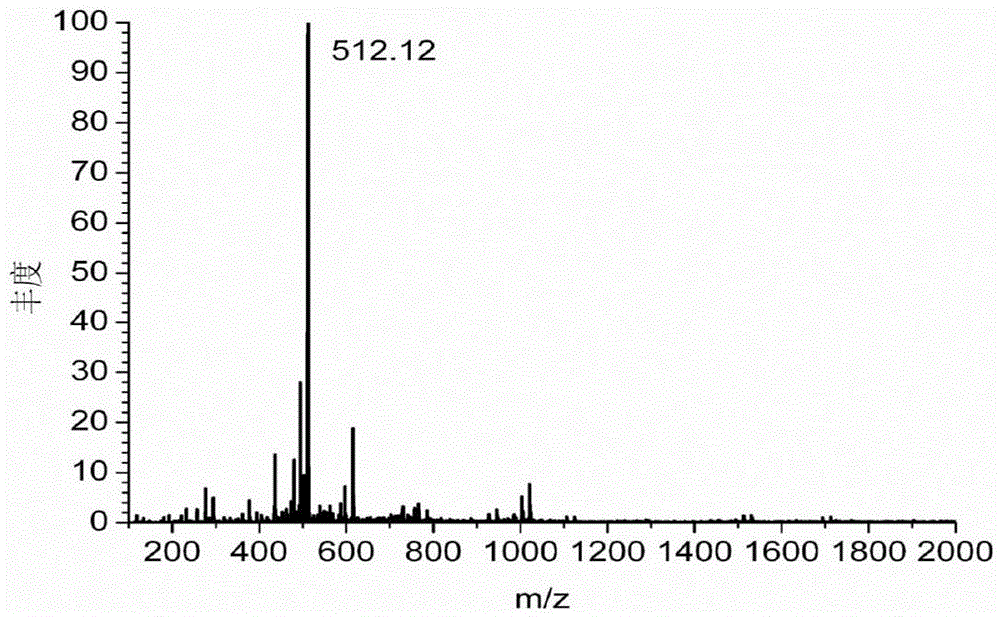
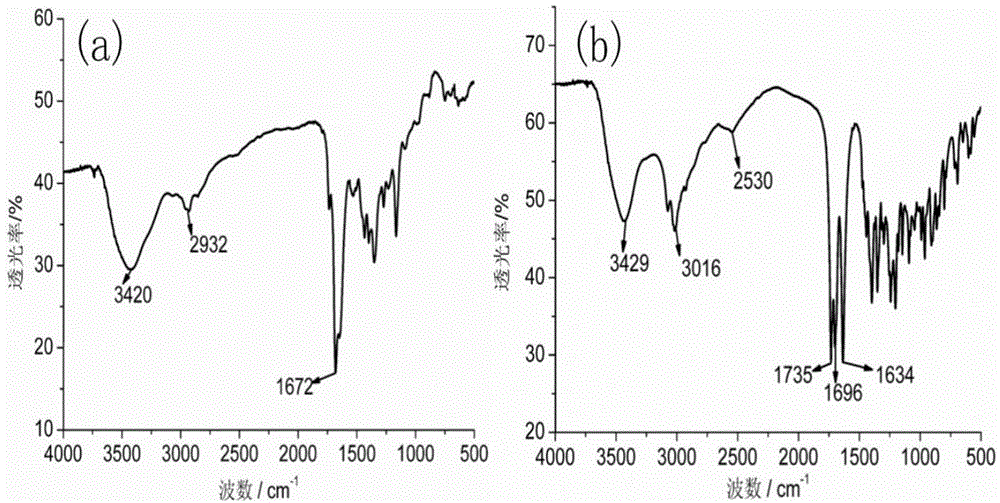
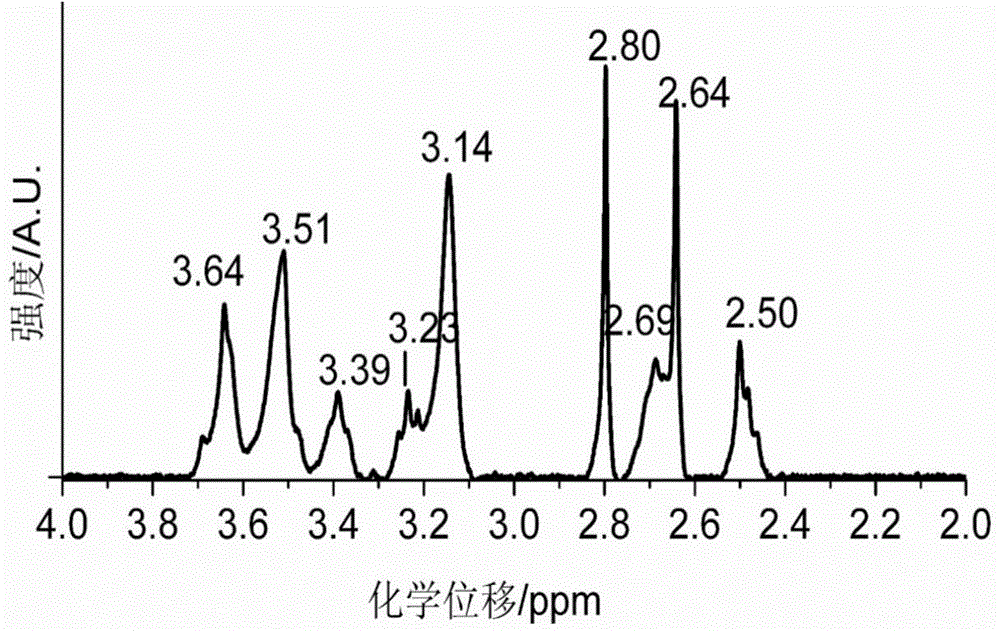
[0129] Dissolve 1g of diethylenetriaminepentaacetic dianhydride in 20mL of N,N-dimethylformamide, and heat to 70°C to obtain solution A. Dissolve 0.55g of 2-aminoethanethiol in 15mL of N,N-dimethylformamide, add 0.87mL of triethylamine, and heat to 70°C to obtain solution B. Add solution B to solution A, heat and stir at 70°C overnight. During the reaction process, the solution gradually changed from colorless to light yellow, indicating that diethylenetriaminepentaacetic dianhydride and 2-aminoethanethiol had undergone amidation reaction to generate a chelating agent with mercapto groups. After the solution was cooled to room temperature, it was placed in an ice bath, and the white precipitate was filtered off. The obtained filtrate was concentrated by rotary evaporation, and a white precipitate was obtained after adding chloroform. The obtained white precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com