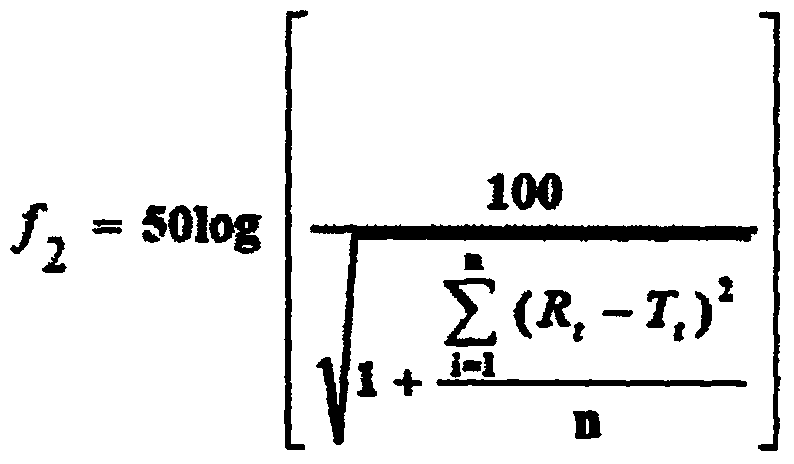Gabapentin tablet and preparation method thereof
A technology for gabapentin tablets and gabapentin is applied in the directions of pill delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of low drug content, undisclosed inclusion of gabapentin, and no research, and achieves content uniformity that meets the requirements of Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
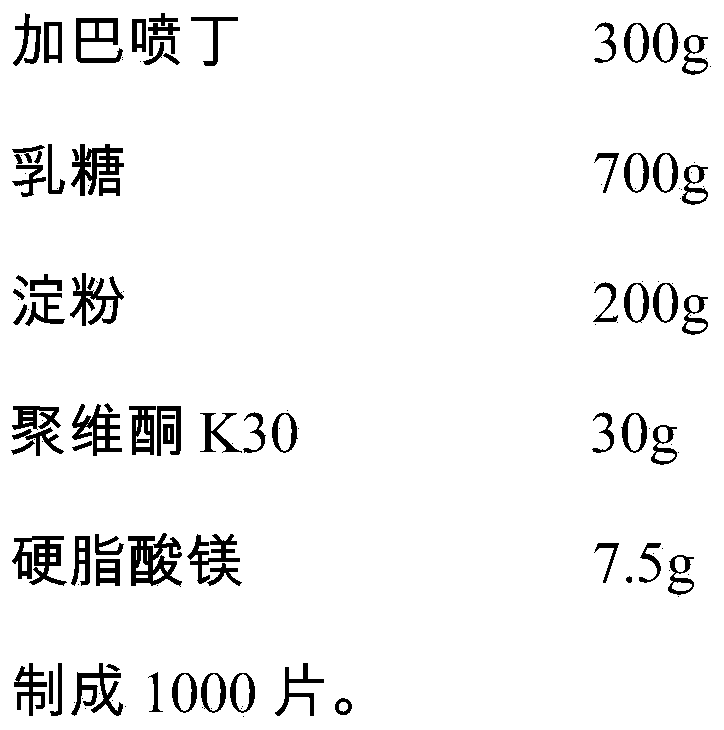
[0029] Gabapentin tablets are prepared from the following medicinal components:
[0030]
[0031] Preparation
[0032] Gabapentin and lactose are micronized at a ratio of 1:20 for use; prepare a 15% concentration of PVP K30 aqueous solution for use; weigh the prescribed amount of gabapentin, lactose, and starch and mix them evenly, add PVP K30 aqueous solution for granulation, dry, and add the corresponding The prescription amount of magnesium stearate is mixed evenly, the content of the mixture is measured, the tablet weight is calculated, and the tablet is pressed to obtain it.
[0033] The prepared gabapentin tablets have the following characteristics:
[0034] Content uniformity inspection: qualified. Disintegration time limit inspection: complete disintegration within 5 minutes, the disintegration phenomenon is the same as the original development.
Embodiment 2
[0036] Gabapentin tablets are prepared from the following medicinal components:
[0037]
[0038] Preparation
[0039] Gabapentin and lactose are micronized at a ratio of 1:20 for use; prepare a 15% concentration of PVP K30 aqueous solution for use; weigh the prescribed amount of gabapentin, lactose, and starch and mix them evenly, add PVP K30 aqueous solution for granulation, dry, and add the corresponding The prescription amount of magnesium stearate is mixed evenly, the content of the mixture is measured, the tablet weight is calculated, and the tablet is pressed to obtain it.
[0040] The prepared gabapentin tablets have the following characteristics: content uniformity inspection: qualified. Disintegration time limit inspection: complete disintegration within 5 minutes.
Embodiment 3
[0042] Gabapentin tablets are prepared from the following medicinal components:
[0043]
[0044]
[0045] Preparation
[0046] Gabapentin and lactose are micronized at a ratio of 1:20 for use; prepare a 15% concentration of PVP K30 aqueous solution for use; weigh the prescribed amount of gabapentin, lactose, and starch and mix them evenly, add PVP K30 aqueous solution for granulation, dry, and add the corresponding The prescription amount of magnesium stearate is mixed evenly, the content of the mixture is measured, the tablet weight is calculated, and the tablet is pressed to obtain it.
[0047] The prepared gabapentin tablets have the following characteristics: content uniformity inspection: qualified. Disintegration time limit inspection: complete disintegration within 5 minutes.
[0048] The specific values of the dissolution profile of each embodiment of the present invention and the reference preparation (commercially available ordinary tablets, Hainan Serike Pharmaceutical,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com