Preparation method of lithium ion secondary battery negative material manganese cobalt oxide
A secondary battery and negative electrode material technology, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of complex preparation methods, voltage hysteresis, and low initial charge and discharge efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
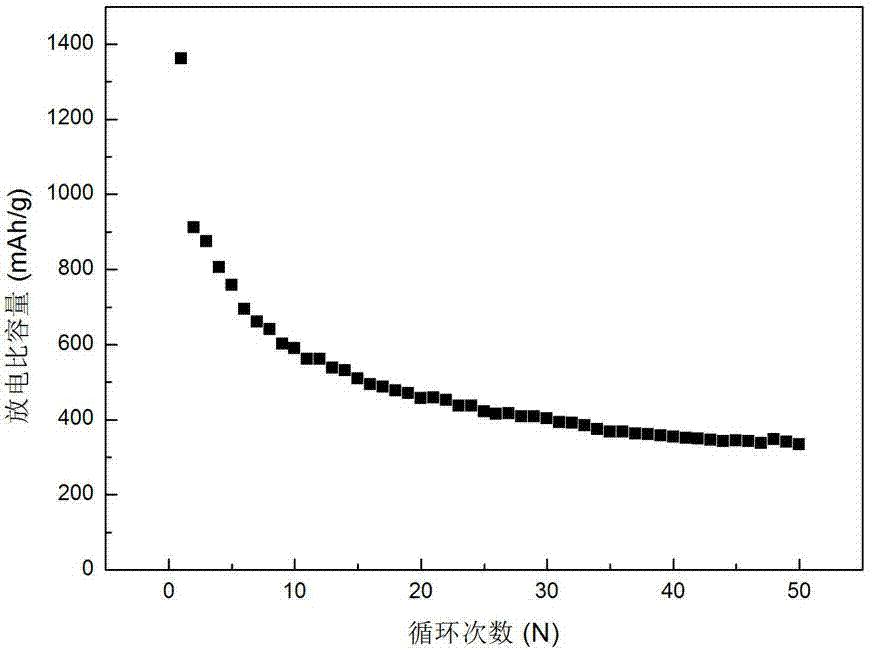
Image
Examples
Embodiment 1
[0023] (1) Dissolve manganese acetate and cobalt acetate in deionized water at 0.01 mol: 0.005 mol, and magnetically stir until completely dissolved; then 0.015 mol-0.030 mol of ethylenediaminetetraacetic acid (EDTA)-polyacrylic acid (PPA) Add a small amount of deionized water to the double chelating agent to moisten, add 0.15 mol (12 mL) ammonia water, shake until a colorless and transparent solution is formed, then add the colorless and transparent double chelating agent EDTA-PPA solution to the inorganic salt solution to form a transparent solution; (2) The obtained solution was dried using a spray dryer with an inlet temperature of 180 °C and an outlet temperature of 100 °C; (3) The solution was injected with a peristaltic pump at a flow rate of 15 mL min -1 ;(4) The nozzle gas flow is controlled by an air compressor pump, and the flow rate is 350 L h -1 (5) The outlet air is evacuated through the outlet filter, and the powder obtained by spray drying is calcined at 800°C ...
Embodiment 2
[0025] (1) Dissolve manganese acetate and cobalt acetate in deionized water at a molar volume of 0.01 mol: 0.005 mol, and add a small amount of deionized chelating agent of 0.015 mol-0.030 mol ethylenediaminetetraacetic acid (EDTA)-citric acid (CA) Wet with water, add 0.15 mol (12 mL) ammonia water, shake until a colorless and transparent solution is formed, then add the colorless and transparent double chelating agent EDTA-CA solution into the inorganic salt solution to form a transparent solution; (2) The obtained solution is sprayed The dryer was used for drying, the inlet temperature was 180 °C, and the outlet temperature was 120 °C; (3) The solution was injected with a peristaltic pump at a flow rate of 20 mL min -1 ;(4) The nozzle gas flow is controlled by an air compressor pump, and the flow rate is 400 L h -1 (5) The outlet air is evacuated through the outlet filter, and the powder obtained by spray drying is calcined at 800 °C to obtain the desired product Mn 2 CoO ...
Embodiment 3
[0027] (1) Dissolve manganese acetate and cobalt acetate in deionized water at 0.01 mol: 0.005 mol, add a small amount of deionized water to 0.015 mol-0.030 mol ethylenediaminetetraacetic acid (EDTA)-acetylacetone double chelating agent , add 0.15 mol (12 mL) ammonia water, shake until a colorless and transparent solution is formed, then add the colorless and transparent double chelating agent EDTA-acetylacetone solution into the inorganic salt solution to form a transparent solution, heat and stir at 80°C until a gel is formed. (2) The obtained solution was dried using a spray dryer with an inlet temperature of 200 °C and an outlet temperature of 120 °C; (3) The solution was injected with a peristaltic pump at a flow rate of 20 mL min -1 ;(4) The nozzle gas flow is controlled by an air compressor pump, and the flow rate is 400 L h -1 (5) The outlet air is evacuated through the outlet filter, and the powder obtained by spray drying is calcined at 800°C to obtain the desired pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com