Disulfide bond-containing polymerizable taxol monomer and synthetic method thereof
A paclitaxel and disulfide bond technology, applied in the field of drug synthesis, can solve the problems of unstable micellar system, increased drug toxicity and side effects, weak interaction force, etc., and achieves reduction of drug burst release, wide application range and simple method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
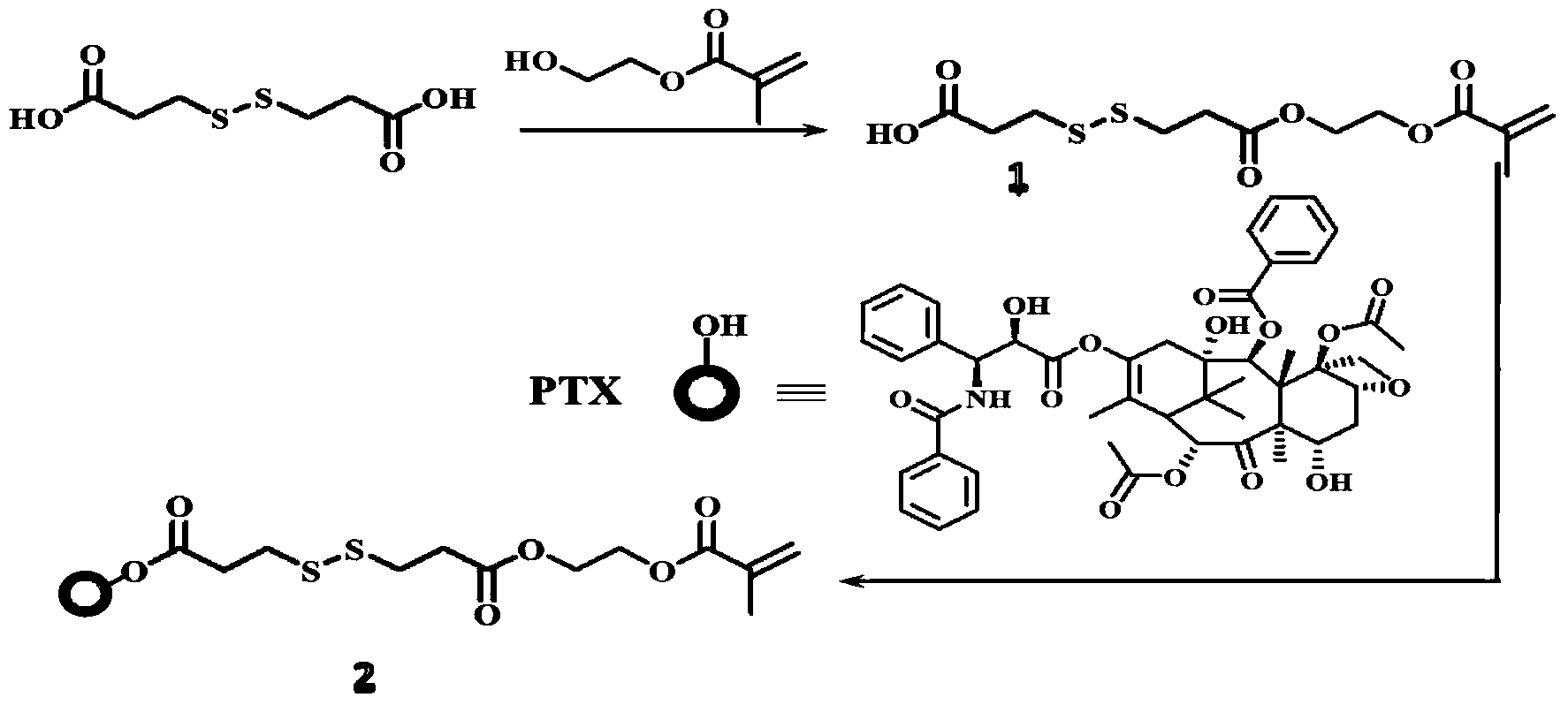
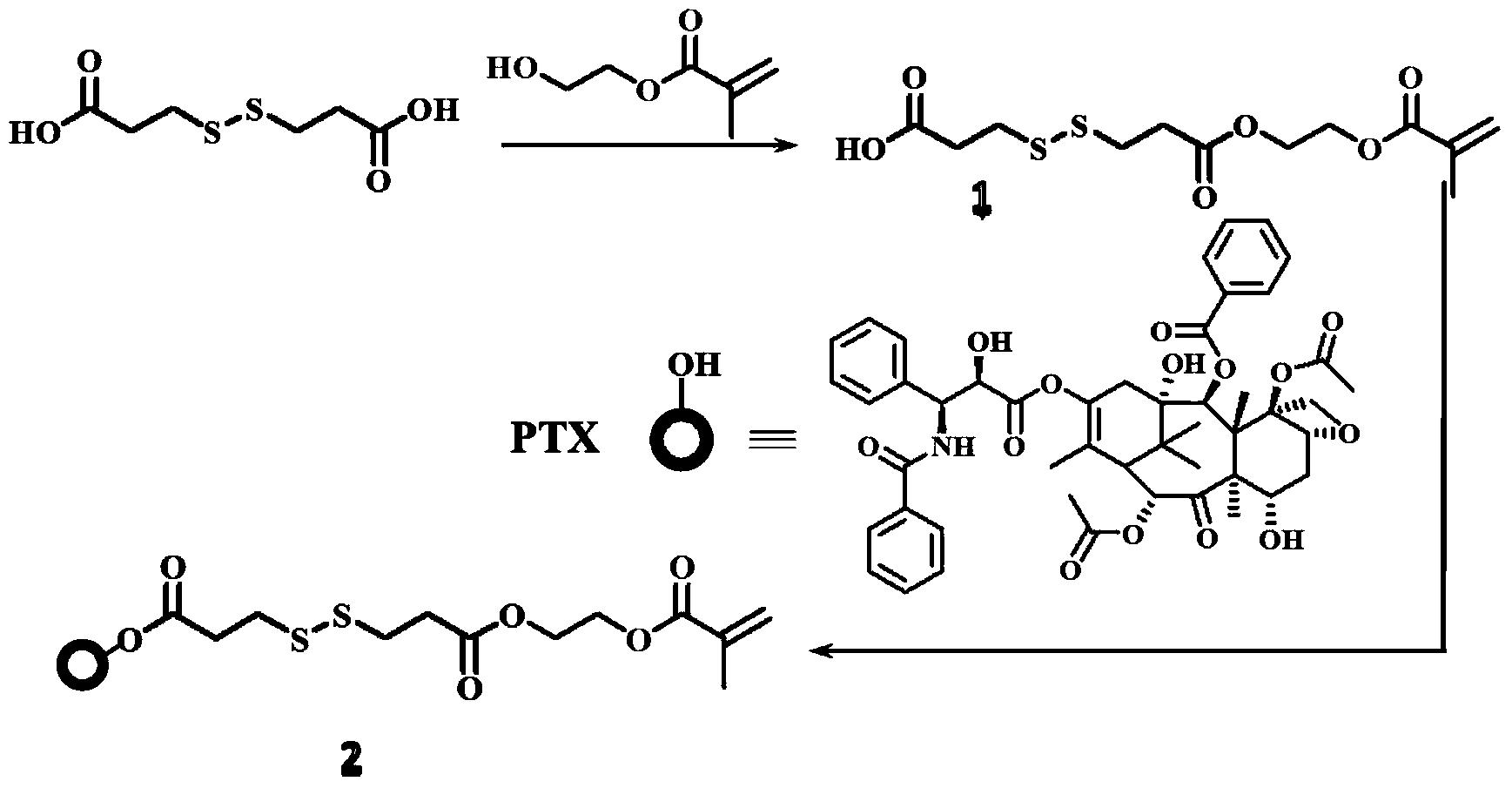
[0018] 4.4g 3,3'-dithiodipropionic acid (DTPA), 0.6mL hydroxyethyl methacrylate (HEMA) and 0.05g 4-dimethylaminopyridine ( DMAP) was dissolved in 50mL tetrahydrofuran (THF), then 0.5mL N,N'-diisopropylcarbodiimide (DIC) was added dropwise to the solution, stirred at room temperature overnight, and the crude product was washed with n-hexane:ethyl acetate=1 DTPA-HEMA (1) was obtained after silica gel column chromatography at :1.
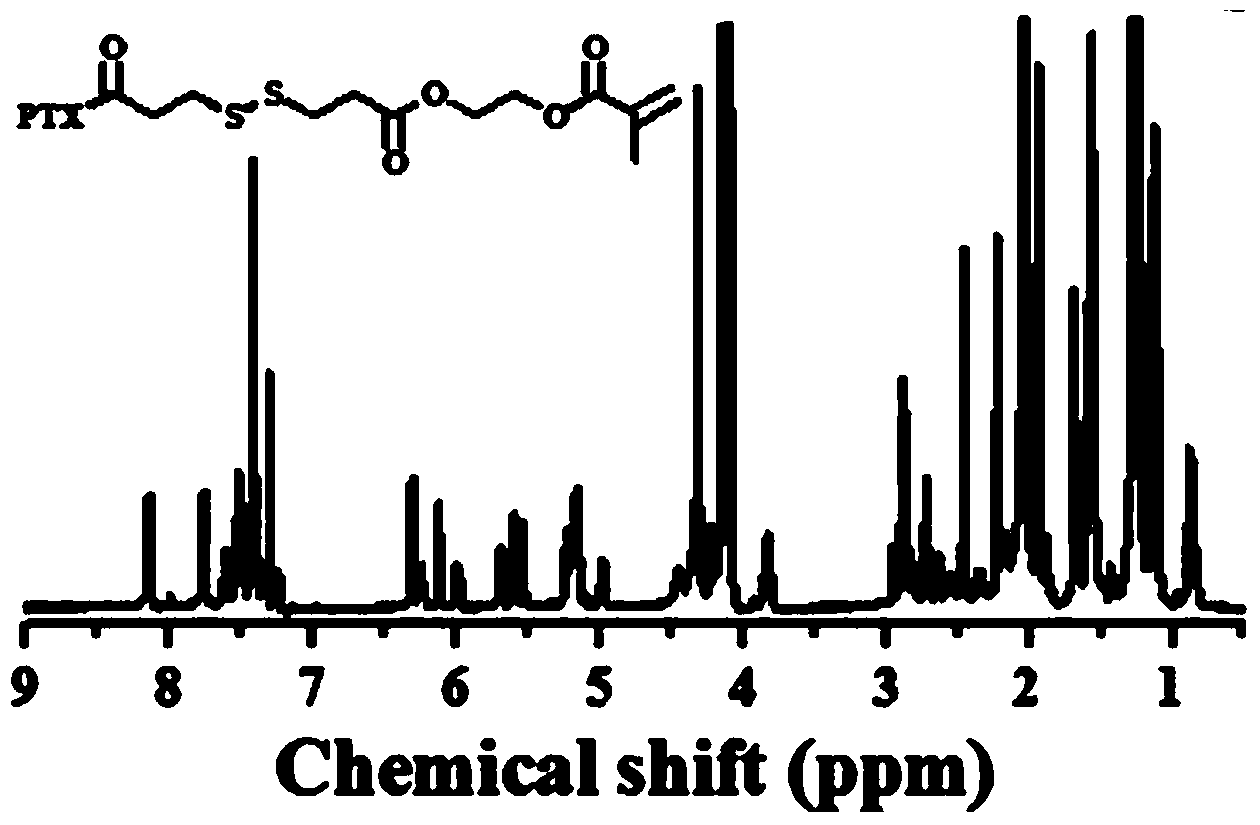
[0019] Add 1g of paclitaxel (PTX), 0.024g of DMAP, 0.78g of DTPA-HEMA into 70mL of dichloromethane (DCM), then dropwise add 23μLDIC, stir the solution at room temperature overnight, and use a silica gel column layer of n-hexane:ethyl acetate=1:1 The crude product was analyzed to obtain PTX-DTPA-HEMA (PTX-linker, 2).
[0020] synthetic route:
[0021]
Embodiment 2
[0023] According to the synthetic route, 6.7g of 3,3'-dithiodipropionic acid, 0.9mL of hydroxypropyl methacrylate and 0.09g of 4-dimethylaminopyridine were dissolved in 100mL of toluene, and then 1.0mL of N,N'-diisopropylcarbodiimide, stirred overnight at room temperature. The crude product was subjected to silica gel column chromatography with n-hexane:ethyl acetate=1:1 to obtain the intermediate product (1).
[0024] Add 1 g of docetaxel, 0.024 g of DMAP, and 0.78 g of intermediate product (1) into 70 mL of dichloromethane, followed by dropwise addition of 23 μL of DIC, and stir the solution overnight at room temperature. The crude product was also treated with silica gel column chromatography with n-hexane: ethyl acetate = 1:1 to obtain disulfide bond-containing polymerizable docetaxel monomer (2).
Embodiment 3
[0026] According to the synthetic route, 7.4g of 3,3'-dithiodibenzoic acid, 1.1mL of 2-hydroxypropyl acrylate and 0.09g of 4-dimethylaminopyridine were dissolved in 100mL of tetrahydrofuran, and then 1.0 mLN,N'-diisopropylcarbodiimide, stirred overnight at room temperature. The crude product was subjected to silica gel column chromatography with n-hexane: ethyl acetate = 1:1 to obtain the intermediate product (1);
[0027] Add 1 g of paclitaxel, 0.024 g of DMAP, and 0.78 g of the intermediate product (1) into 70 mL of dichloromethane, then add 23 μL of DIC dropwise, and stir the solution overnight at room temperature. The crude product was also treated with silica gel column chromatography with n-hexane: ethyl acetate = 1:1 to obtain disulfide bond-containing polymerizable paclitaxel monomer (2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com