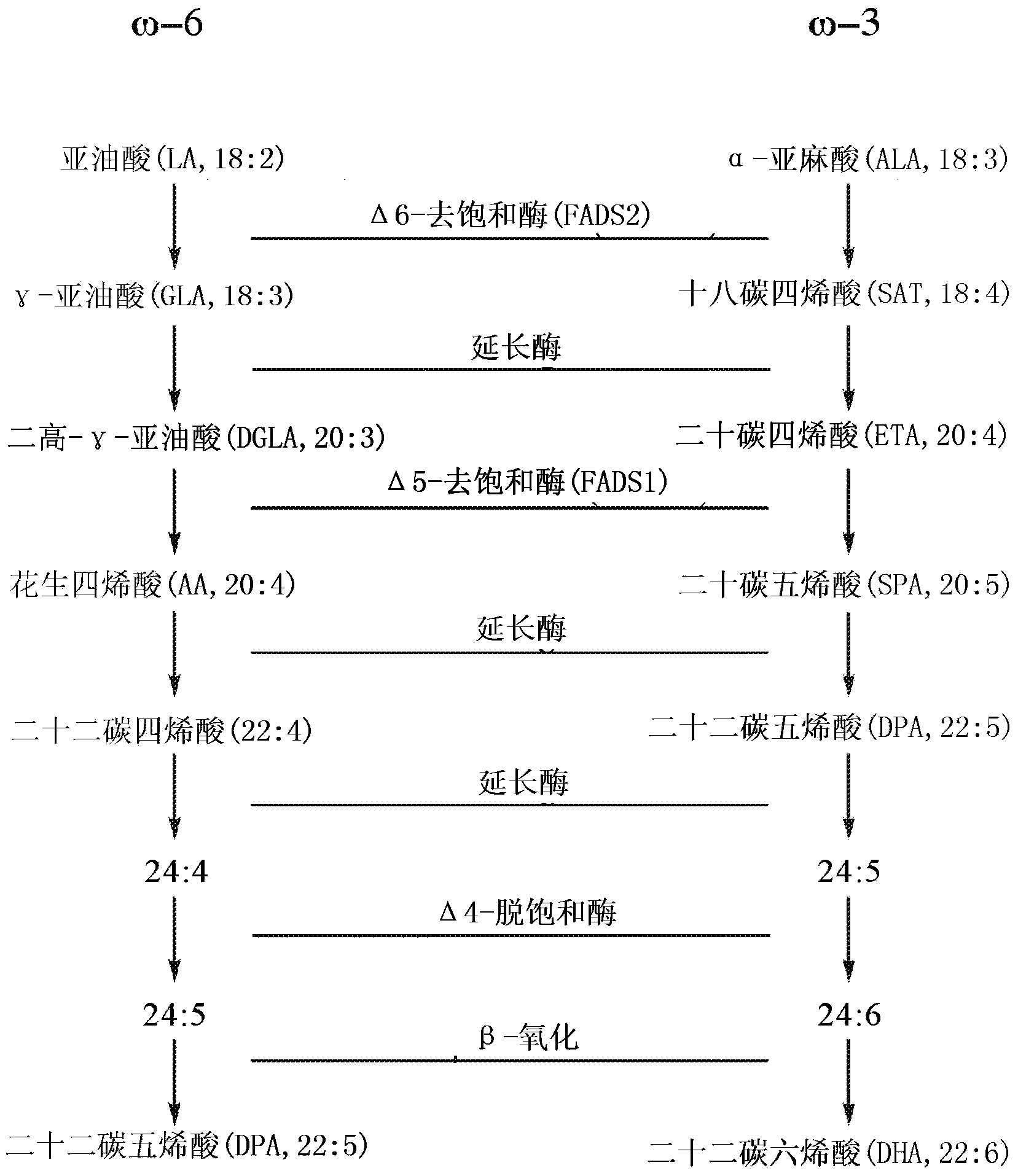
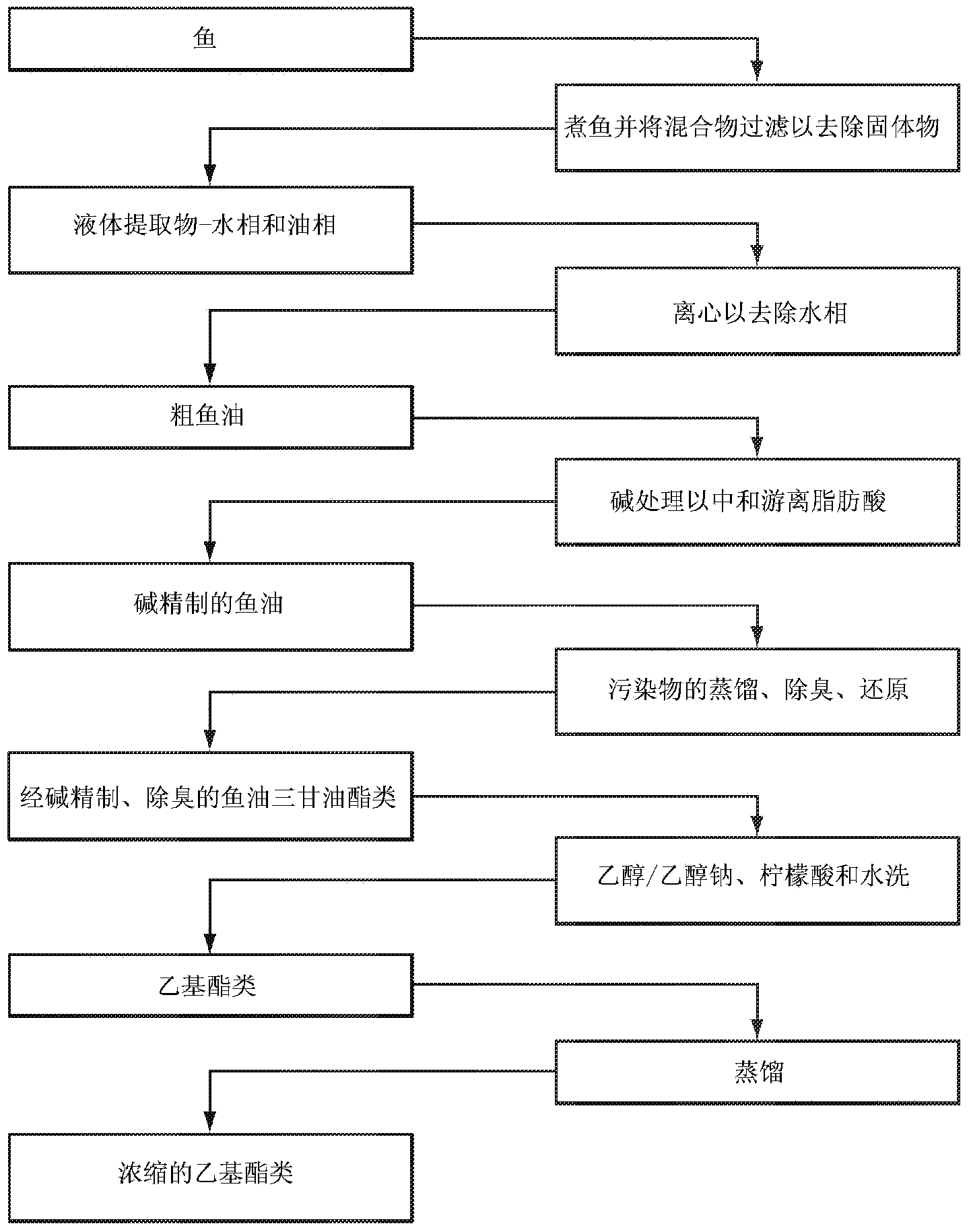
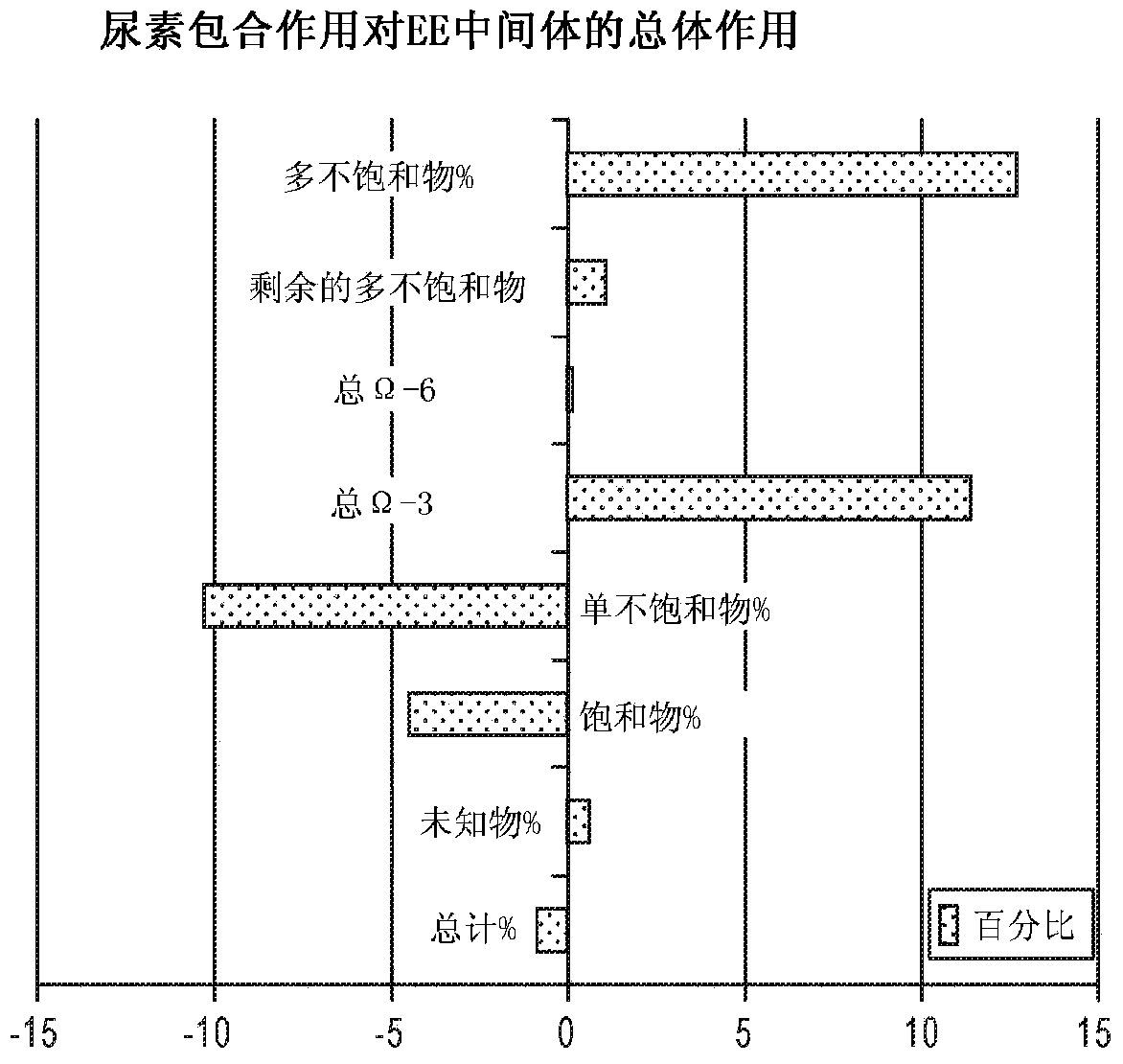
DPA-enriched compositions of omega-3 polyunsaturated fatty acids in free acid form
A technology of unsaturated fatty acids and compositions, which is applied in the direction of drug combinations, active ingredients of anhydrides/acids/halides, medical preparations of non-active ingredients, etc., and can solve problems such as the increase of ethyl carbamate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7
[0094] Example 7 presents the results of the ECLIPSE clinical trial, an open-label, single-dose, randomized 4-way crossover study of The bioavailability of the DPA-enriched pharmaceutical composition (hereinafter referred to as ) bioavailability were compared. 1 gram each according to FDA-approved product label The capsules contain at least 900 mg of ethyl esters of omega-3 fatty acids derived from fish oil, mainly eicosapentaenoic acid (EPA, approximately 465 mg) and docosahexaenoic acid (DHA, approximately 375mg) of the combination of ethyl esters. used in this test The batch contained 57.3% (a / a) EPA, 19.6% (a / a) DHA, and 6.2% (a / a) DPA, each substantially in the free acid form.
[0095] During the high-fat diet period, use The baseline-adjusted changes in the total EPA+DHA and individual EPA and DHA absorption curves (AUC) (with omega-3 PUFA in the free acid form) were significantly greater than those using (omega-3-PUFA ethyl esters) and was significantly bett...
Embodiment 8
[0097] The results from the 14-day bioavailability study are presented in Example 8, showing that the increase in bioavailability observed in the single-dose ECLIPSE trial was maintained, and even enhanced, over the 2-week dosing period. In addition, disaggregated subject-specific data suggest that Subjects with minimal responses still had the same Subjects with the best response compared to greater 14-day EPA+DHAC max .
Embodiment 10
[0098] Example 10 presents the results of the EVOLVE trial, a 12-week, double-blind, Controlled study of olive oil. The primary study endpoint was the percent change in plasma triglyceride levels from baseline to end of treatment (EOT). The secondary study endpoint was the percent change in plasma non-high-density lipoprotein cholesterol (non-HDL-C) from baseline to EOT.
[0099] As seen in Figures 20-23, using The 12-week treatment of ® resulted in a significant increase in the plasma levels of EPA, DHA and DPA.
[0100] The increase in plasma levels of EPA, DHA, and DPA was accompanied by a significant decrease in plasma AA levels, and the 4g dosing regimen achieved an 18% mean reduction, a 25.9% median reduction, and a least squares (LS) mean of 23.2% decline. These plasma arachidonic acid levels were observed to decrease despite the administration of exogenous arachidonic acid, the arachidonic acid used in this trial It exists at the content of 2.446% (a / a) in the b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com