Method for modifying lithium cobalt oxide material
A unique lithium cobalt oxide, soluble technology, applied in the field of modified lithium cobalt oxide materials, can solve the problems of poor resistance to overcharge, poor cycle performance of lithium cobalt oxide, etc., to improve cycle performance, improve rate performance, and enhance application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: LiCoO 2 -Li 0.35 La 0.55 TiO 3 (5wt%) synthesis, structural stability test and electrochemical performance test of a simulated battery assembled with Li in the voltage range of 2.7-4.2V.
[0022] Weigh lithium nitrate, lanthanum nitrate, and tetrabutyl titanate in a certain molar ratio, and obtain Li 0.35 La 0.55 TiO 3 Sol, with LiCoO 2 Mix, Li 0.35 La 0.55 TiO 3 accounted for LiCoO 2 5% of the mass, stirred at 100°C for 1 hour, then baked in an oven at 250°C for 5 hours, then baked in a muffle furnace at 800°C, holding time for 5 hours, and the heating rate was 5°C min -1 , that is, to get the required LiCoO 2 -Li 0.35 La 0.55 TiO 3 (5 wt%) composite material.
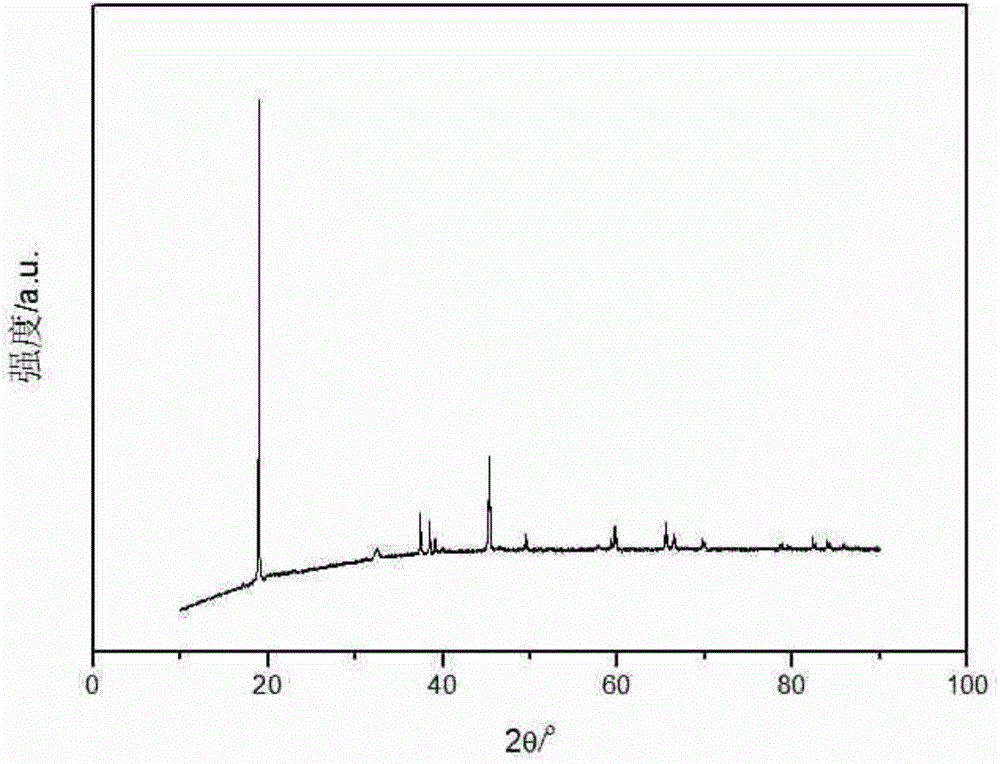
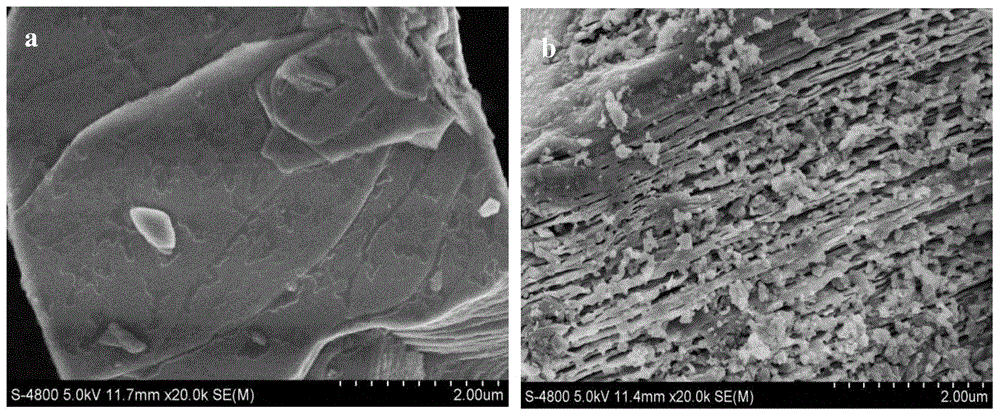
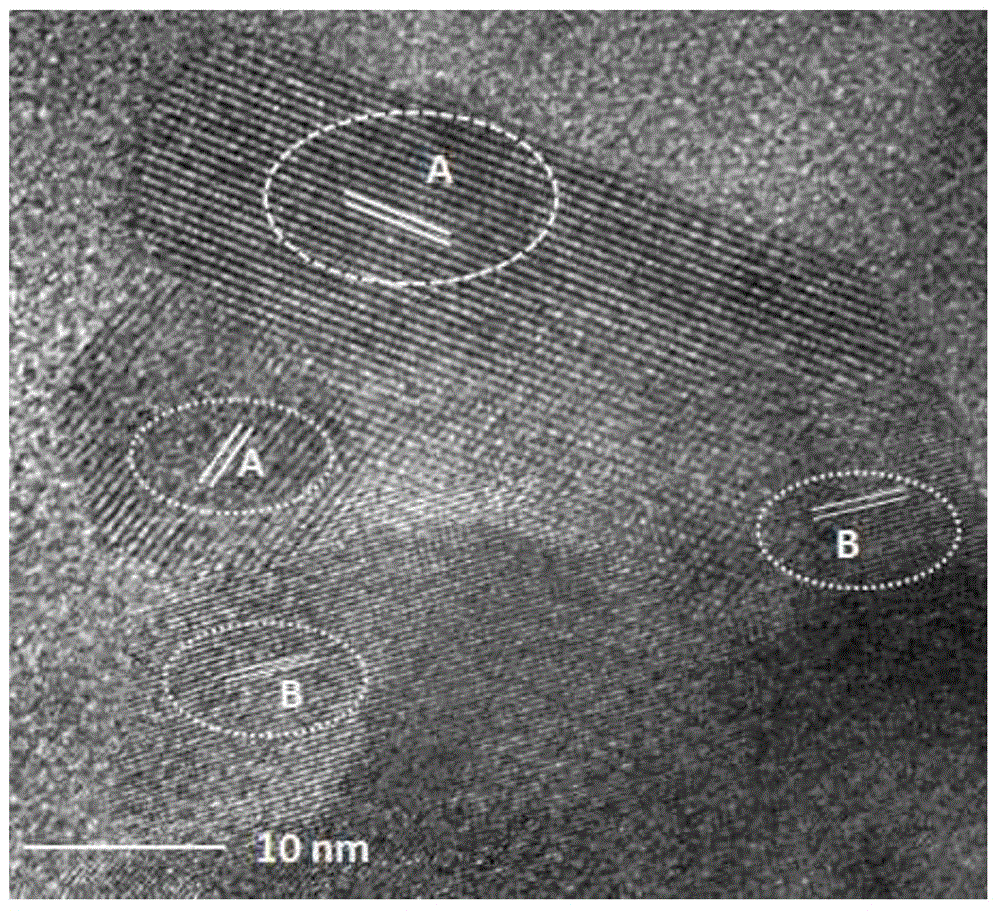
[0023] XRD powder diffraction method such as figure 1 shown, indicating 5wt% L 0.35 With the addition of LTO, no phase change occurred. The microstructure of the product was observed by SEM, such as figure 2 As shown in a, it is LiCoO 2 The SEM picture, as can be seen from the fi...
Embodiment 2
[0026] Example 2: LiCoO 2 -Li 0.35 La 0.55 TiO 3(0.05wt%) and Li assembled to simulate the electrochemical performance test of the battery in the voltage range of 2.7-4.2V.
[0027] Weigh lithium acetate, lanthanum oxide, titanium tetrachloride in a certain molar ratio, dissolve lanthanum oxide with nitric acid to obtain a lanthanum solution, and then pass through the EDTA-CA method (refer to M.Vijayakumar, Y.Inaguma et al.Chem.Mater.16 (2004) 2719-2724) prepared Li 0.35 La 0.55 TiO 3 Sol, with LiCoO 2 Mix, Li 0.35 La 0.55 TiO 3 accounted for LiCoO 2 0.05% of the mass, stir at 40°C for 5 hours, then bake in an oven at 180°C for 12 hours, then bake in a muffle furnace at 700°C, hold for 8 hours, and heat up at a rate of 10°C min -1 , that is, to get the required LiCoO 2 -Li 0.35 La 0.55 TiO 3 (0.05 wt%) composite material.
[0028] XRD powder diffraction method such as Image 6 shown, indicating that 0.05wt% Li 0.35 La 0.55 TiO 3 The addition of , no phase c...
Embodiment 3
[0029] Example 3: LiCoO 2 -Li 0.75 La 0.42 TiO 3 (2.5wt%) and Li are assembled to simulate the electrochemical performance test of the battery in the voltage range of 2.7-4.2V.
[0030] Lithium carbonate, lanthanum nitrate, and tetrabutyl titanate are weighed in a certain molar ratio, and Li 0.75 La 0.42 TiO 3 Sol, with LiCoO 2 Mix, Li 0.75 La 0.42 TiO 3 accounted for LiCoO 2 2.5% of the mass, stirred at 80°C for 2 hours, then baked in an oven at 200°C for 6 hours, then baked in a muffle furnace at 750°C, holding time for 6 hours, and the heating rate is 6°C min -1 , that is, to get the required LiCoO 2 -Li 0.75 L a0.42 TiO 3 (2.5 wt%) composite material.
[0031] XRD powder diffraction method such as Figure 7 shown, indicating that 2.5wt% Li 0.75 La 0.42 TiO 3 addition, no phase change occurred. A simulated battery was assembled according to the method of Example 1. Investigate the charge-discharge cycle performance on a high-precision battery tester. I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com