Carbene-diazo compound-olefine aldehyde terpolymer and application of carbene-diazo compound-olefine aldehyde terpolymer as bidirectional conversion fluorescent material and anti-cancer drug
A technology of terpolymers and diazo compounds, which is applied in the fields of polymer materials and biomedicine to achieve the effects of improving sensitivity, enhancing fluorescence intensity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Cinnamaldehyde (1.32 g, 0.01 mol) and methyl diazoacetate (MDA) (1.00 g, 0.001 mol) were added to a 10 ml round bottom flask connected to a safety bottle containing mineral oil. React at room temperature for 120 h, cool to room temperature after completion, reprecipitate with chloroform / ether, and dry in vacuo to obtain a polymer.
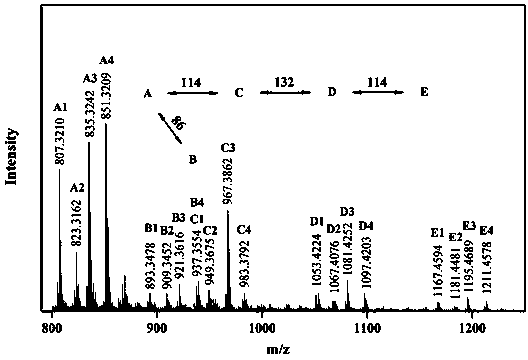
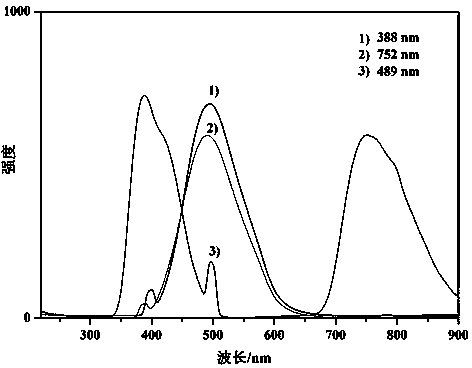
[0040] figure 1 It is the time-of-flight mass spectrum of the polymer obtained in Example 1. From the spectrogram, it can be resolved that the polymer structure is a terpolymer of carbene, diazo compound and cinnamaldehyde. figure 2 It is its fluorescence spectrum. Fluorescence can be obtained from ultraviolet and near-infrared light excitation, and the fluorescence intensity obtained by near-ultraviolet excitation is not much different from that of ultraviolet excitation, which has a good up-conversion fluorescence effect.
Embodiment 2
[0042] Cinnamaldehyde (1.32 g, 0.01 mol) and ethyl diazoacetate (2.28 g, 0.02 mol) were added to a 10 ml round bottom flask connected to a safety bottle containing mineral oil. Heat to 150° C. for 60 minutes, cool to room temperature after the reaction, reprecipitate with chloroform / n-hexane, and vacuum dry to obtain a polymer.
Embodiment 3
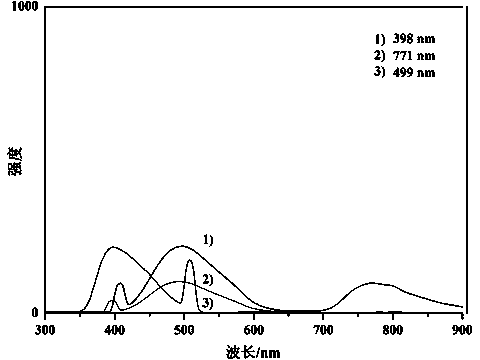
[0044] Add trans-crotonaldehyde (0.70g, 0.01mol) and ethyl diazoacetate (0.57g, 0.005mol) into a 10ml round bottom flask, use toluene as solvent reflux, and the condenser tube is connected to a safety bottle equipped with mineral oil, After reacting for 2 minutes, cool to room temperature, reprecipitate with chloroform / n-hexane, and dry in vacuum to obtain a polymer. image 3 It is its fluorescence spectrum, which can obtain fluorescence from ultraviolet and near-infrared light excitation, and has a good up-conversion fluorescence effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com