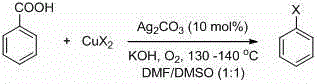
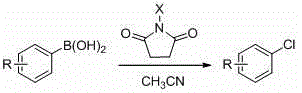
Ortho-position mono chlorine substituted N-aryl azaindole compound and synthetic method thereof
An aryl nitrogen compound technology, which is applied in the field of N-aryl azaindole and its synthesis, can solve the problems of environmental pollution, high toxicity of halogenated reagents, harsh reaction conditions, etc., and achieves good development prospects, good reactivity, Moderate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: 1-(2-chlorophenyl)-1 H -pyrrole[2,3- b ] The preparation of pyridine
[0044] 1-(2-Chlorophenyl)-4-1 H -pyrrole[2,3- b ] pyridine adopts the following steps: 1. add 19.42 grams of 1-phenyl-1H-pyrrole [2,3- b ]pyridine, 0.62 g [RhCp * Cl 2 ] 2 , 58.00 g of copper trifluoroacetate, 7.40 g of lithium carbonate, 16.60 g of tert-butylisonitrile, 500 ml of 1,2-dichloroethane, heated to 130-140 °C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add 3 mol / L ammonia solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain the crude product ; ③ The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 20: 1) to obtain 17.61 grams of 1-(2-chlorophenyl)-4-1 H -pyrrole[2,3- b ] Pyridine, the productive rate is 77%.
[0045] –1 ): 3064, 1587, 1514, 1422, 1357, 1323, 1279, 797, 773,...
Embodiment 2
[0050] Example 2: 1-(2-chloro-4-methylphenyl)-1 H -pyrrole[2,3- b ] The preparation of pyridine
[0051] 1-(2-Chloro-4-methylphenyl)-1 H -pyrrole[2,3- b ] pyridine adopts the following steps: 1. add 20.83 grams of 1-(p-tolyl)-1 in 1000 milliliters of reactor H -pyrrole[2,3- b ]pyridine, 0.74 g [RhCp * Cl 2 ] 2 , 66.58 grams of copper trifluoroacetate, 8.88 grams of lithium carbonate, 19.09 grams of tert-butylisonitrile, 500 milliliters of 1,2-dichloroethane, heated to 130-140 °C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add 3 mol / L ammonia solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain the crude product ; ③ The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 20: 1) to obtain 17.23 grams of 1-(2-chloro-4-methylphenyl)-1 H -pyrrole[2,3- b ] Pyridine, the productive rate...
Embodiment 3
[0057] Embodiment three: 3-chloro-4-(1 H -pyrrole[2,3- b ]pyridine) the preparation of methyl benzoate
[0058] 1-(2-Chloro-4-methylphenyl)-1 H -pyrrole[2,3- b ] pyridine) methyl benzoate adopts the following steps: 1. add 25.23 grams of 4-(1 H -pyrrolo[2,3-b]pyridine) methyl benzoate, 0.86 g [RhCp * Cl 2 ] 2 , 69.48 grams of copper trifluoroacetate, 9.62 grams of lithium carbonate, 19.92 grams of tert-butylisonitrile, 500 milliliters of 1,2-dichloroethane, heated to 130-140 °C. Use thin layer chromatography to track the reaction until the reaction raw materials disappear; ② After the reaction, add 3 mol / L ammonia solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain the crude product ; ③The crude product was purified by column chromatography (petroleum ether: ethyl acetate=20: 1) to obtain 20.36 grams of 3-chloro-4-(1 H -pyrrole[2,3- b ] pyridine) methyl benzoate, the productive rate is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com