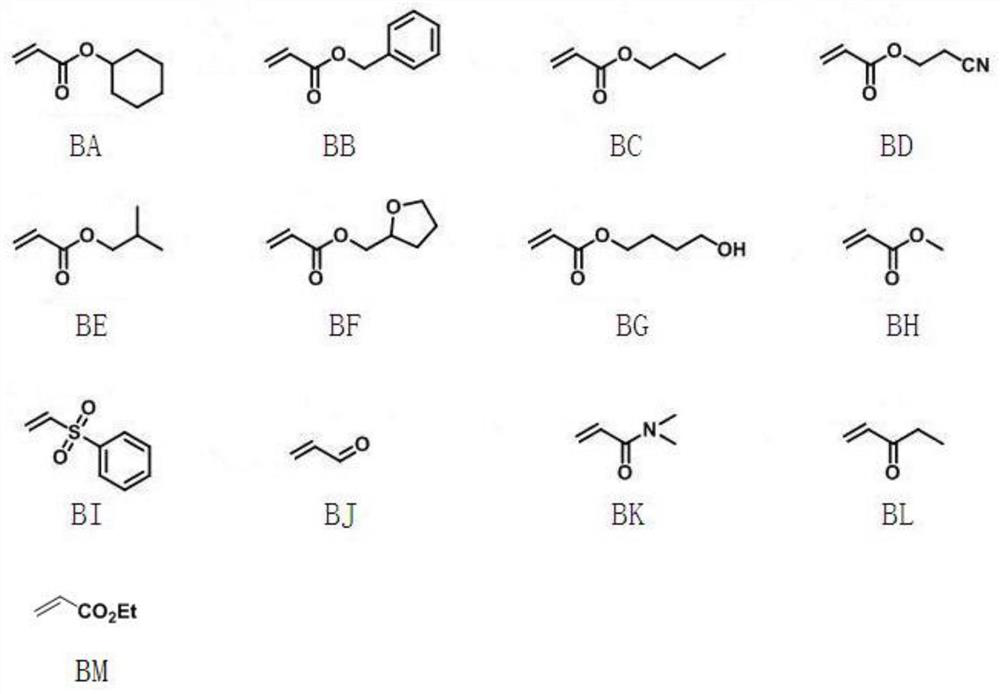
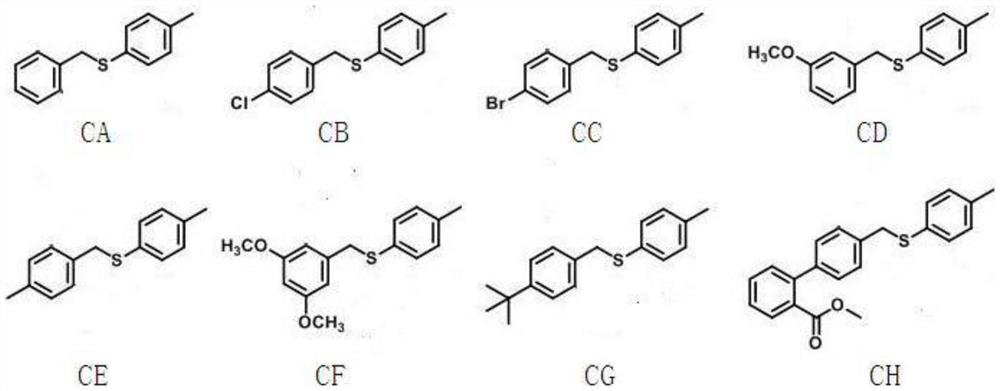
Process for the preparation of aromatic alkenyl compounds
A technology of aromatic alkenes and compounds, applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of high catalyst consumption, poor reaction selectivity, high reaction temperature, etc., and achieve the effect of good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention also provides a kind of preparation method of thioether:
[0061] Step A: Under air atmosphere at room temperature, add benzyl bromide (10.0mmol), thiophenol (10.0mmol) and K2CO3 (1.52g, 11.0mmol) in DMF (10.00mL) to a 50mL flask, and keep the mixture at This temperature continues to stir reaction 4h;
[0062] Step B: 15 mL of water was added to the reaction mixture, while ethyl acetate (25 mL) was added and stirred for 5 min, the upper layer was washed with brine three times, and dried over anhydrous sodium sulfate;
[0063] Step C: Evaporate the solvent under reduced pressure, and subject the residue to silica gel column chromatography to obtain thioether.
[0064] It should be pointed out that the substances used in the specific embodiments of the present invention can be purchased from the market. As long as the structural formula is the same, they can be applied to the preparation method of the present invention. Since no improvement is invol...
Embodiment 1
[0066]
[0067] Under an air atmosphere at 80°C, 0.4 mmol of thioether with the structural formula AA, 0.8 mmol of ethyl acrylate, [Cp*IrCl 2 ] 2 0.006mmol, AgBF 4 0.024mmol and Cu(OAc) 2 0.48mmol of the mixture was added to 2mL of hexafluoroisopropanol and stirred for 12h. The reaction mixture was cooled to room temperature and filtered through diatomaceous earth. The solvent was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography (ethyl acetate / petroleum ether =1:10, v / v), to obtain colorless liquid (E)-3-(3-methyl-2-((p-tolylthio)methyl)phenyl) ethyl acrylate, the compound is mono Alkenylated product, 85% yield. 1 H NMR (400MHz, CDCl 3 , TMS) δ7.94 (d, J = 16.0Hz, 1H), 7.36 (dd, J1 = 6.8Hz, J2 = 7.6Hz, 1H), 7.29-7.26 (m, 2H), 7.21-7.15 (m, 2H ), 7.07(d, J=8.0Hz, 2H), 6.22(d, J=15.6Hz, 1H), 4.25(q, J=7.2Hz, 2H), 4.15(s, 2H), 2.41(s, 3H ), 2.32(s, 3H), 1.33(t, J=7.2Hz, 3H). 13 C NMR (100MHz, CDCl 3 )δ 166.7, 142.1...
Embodiment 2
[0069]
[0070] Under an air atmosphere at 80°C, 0.4 mmol of thioether, 0.8 mmol of ethyl acrylate, [Cp*IrCl 2 ] 2 0.006mmol, AgBF 4 0.024mmol and Cu(OAc) 2 0.48mmol of the mixture was added to 2mL of hexafluoroisopropanol and stirred for 12h. The reaction mixture was cooled to room temperature and filtered through diatomaceous earth. The solvent was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography (ethyl acetate / petroleum ether =1:15, v / v), to obtain colorless liquid (E)-3-(5-chloro-2-((p-tolylthio)methyl)phenyl) ethyl acrylate, the compound is monoene The ylated product was obtained with a yield of 58%. 1 H NMR (400MHz, CDCl 3,TMS)δ7.86(d,J=16Hz,1H),7.49(d,1H),7.21-7.19(m,3H),7.09-7.04(q,3H),6.29(d,J=15.6Hz, 1H), 4.26(q, J=6.8Hz, 2H), 4.06(s, 2H), 2.31(s, 3H), 1.34(t, J=6.8Hz, 3H). 13 CNMR (100MHz, CDCl 3 )δ 166.3, 139.9, 137.7, 135.3, 135.2, 133.4, 132.6, 131.9, 130.7, 129.7, 129.6, 126.5, 121.1, 60.6, 37.5, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com