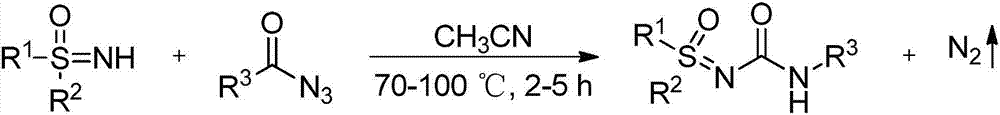
High-efficiency preparation method for sulfoxide sulfinyl urea compounds
A sulfoxide sulfinyl urea and compound technology, which is applied in the field of high-efficiency preparation of sulfoxide sulfinyl urea series compounds, can solve the problems of unstable chemical properties of isocyanates, highly toxic properties, easy hydrolysis and deterioration, and the like. It is beneficial to separation and purification, good reactivity and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
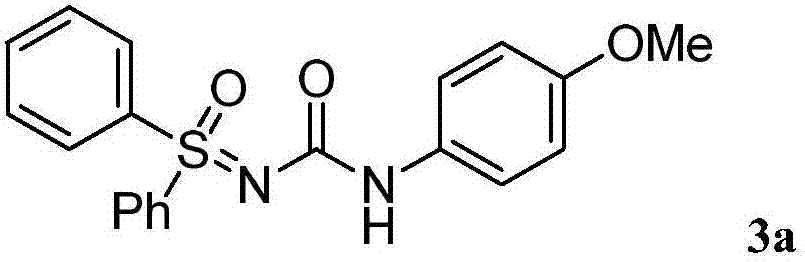
[0016] Dissolve 4-methoxybenzoyl azide (63.7mg, 0.36mmol) in organic solvent acetonitrile (1mL) under air atmosphere, add dropwise to diphenylsulfinimide at 80°C in a magnetic stirrer (65.1mg, 0.3mmol) in acetonitrile (1mL) solution, the reaction was continued for 3 hours under air atmosphere, and the reaction was monitored by TLC plate until complete reaction. After treatment, the pure product 1-sulfinyl-3-phenylurea compound 3a was separated by flash column chromatography. Yield: 96%. The following is the NMR experimental data of product 3a:
[0017] 1 H NMR (400MHz, d6-DMSO) δ9.50(s, 1H), 8.03(d, J=6.8Hz, 4H), 7.63(d, J=7.7Hz, 6H), 7.42(d, J=8.6Hz ,2H),6.81(d,J=8.2Hz,2H),3.68(s,3H).
[0018] 13 C NMR (100MHz, d6-DMSO) δ157.3, 154.8, 140.6, 133.8, 130.2, 127.9, 120.4, 120.0, 114.2, 55.6.
Embodiment 2
[0020]
[0021] Under air atmosphere, 4-methylbenzoyl azide (58 mg, 0.36 mmol) was dissolved in organic solvent acetonitrile (1 mL), and added dropwise to diphenylsulfinimide (65.1 mg, 0.3 mmol) in acetonitrile (1 mL) solution, the reaction was continued for 3 hours under the air atmosphere, and the reaction was monitored by TLC plate during the reaction until complete reaction. After treatment, the pure product 1-sulfinyl-3-phenylurea compound 3b was separated by flash column chromatography. Yield: 91%. The following is the NMR experimental data of product 3b:
[0022] 1H NMR(400MHz,d6-DMSO)δ9.55(s,1H),8.03(d,J=7.1Hz,4H),7.71-7.59(m,6H),7.39(d,J=8.3Hz,2H) ,7.02(d,J=8.1Hz,2H),2.21(s,3H).
[0023] 13 C NMR (100MHz, d6-DMSO) δ157.3, 140.5, 138.2, 133.8, 131.1, 130.2, 129.4, 128.0, 118.6, 20.8.
Embodiment 3
[0025]
[0026] Benzoyl azide (882mg, 6mmol) was dissolved in organic solvent acetonitrile (10mL) under air atmosphere, and added dropwise to diphenylsulfinimide (1.085g, 5mmol) in a magnetic stirrer at 80°C In an acetonitrile (10 mL) solution, the reaction was continued for 3 hours under an air atmosphere, and the reaction was monitored by a TLC plate during the reaction until complete reaction. After treatment, the pure product 1-sulfinyl-3-phenylurea compound 3c was separated by flash column chromatography, and the yield was 87%. The following is the NMR experimental data of product 3c:
[0027] 1 H NMR(400MHz,d6-DMSO)δ9.65(s,1H),8.04(d,J=7.2Hz,4H),7.69-7.62(m,6H),7.52(d,J=8.0Hz,2H) ,7.22(t,J=7.7Hz,2H),6.93(t,J=7.2Hz,1H).
[0028] 13 C NMR (100MHz, d6-DMSO) δ157.4, 140.7, 140.5, 133.9, 130.2, 129.0, 128.0, 122.3, 118.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com