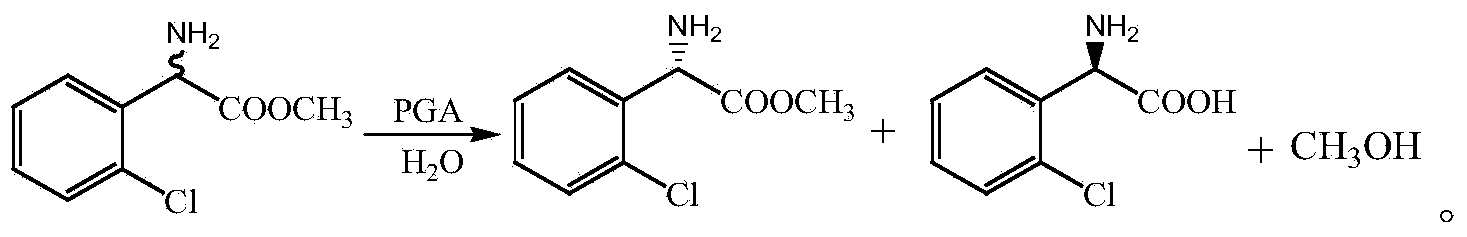
Method for preparing (S)-2-chlorophenylglycine methyl ester single enantiomer by virtue of biological enzyme catalysis
A chlorophenylglycine methyl ester, a catalytic preparation technology, applied in the field of medicine, can solve the problems of expensive chiral resolving agent, complicated operation process, low production efficiency, etc., and achieve simple and easy control of the operation process, low cost and low emission Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In reactor, add 5.0g racemic 2-chlorophenylglycine methyl ester and 50mL pH 7.0, 0.2mol / L sodium phosphate buffer solution, now the concentration of racemic 2-chlorophenylglycine methyl ester is 100g / L, Then add 0.1 g of immobilized penicillin G acylase IPA750. At this time, the mass ratio of immobilized penicillin G acylase to 2-chlorophenylglycine methyl ester is 1:50. The temperature of the water bath is controlled at 30°C and the speed is controlled at 300r. / min, reacted for 30 hours, and finally separated the immobilized enzyme.
[0027] The reactor can adopt the batch reactor commonly used in industry.
Embodiment 2
[0029] In reactor, add 5.0g racemic 2-chlorophenylglycine methyl ester and 50mL pH 7.0, 0.2mol / L sodium phosphate buffer solution, now the concentration of racemic 2-chlorophenylglycine methyl ester is 100g / L, Then add 0.2 g of immobilized penicillin G acylase IPA750, at this time the mass ratio of immobilized penicillin G acylase to 2-chlorophenylglycine methyl ester is 2:50, the temperature of the water bath is controlled at 30°C, and the control speed is 300r / min, reacted for 20 hours, and finally separated the immobilized enzyme.
Embodiment 3
[0031] Add 5.0g racemic 2-chlorophenylglycine methyl ester and 50mL pH 7.0, 0.01mol / L sodium phosphate buffer solution in reactor, the concentration of racemic 2-chlorophenylglycine methyl ester is 100g / L now, Then add 0.1 g of immobilized penicillin G acylase IPA750. At this time, the mass ratio of immobilized penicillin G acylase to 2-chlorophenylglycine methyl ester is 1:50. The temperature of the water bath is controlled at 30°C and the speed is controlled at 300r. / min, reacted for 30 hours, and finally separated the immobilized enzyme.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com