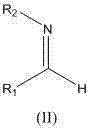
Method for catalyzing and synthesizing optically pure beta-nitramine derivative
A technology of derivatives and nitramines, applied in the field of synthesis of β-nitroamines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of (S)-1-nitro-2-(4-nitrophenyl)-2-tert-butoxyamide ethane
[0034] Add 0.49 g (1 mmol) of chiral copper complex (catalyst) and 40 mL of absolute ethanol as a solvent into a 100 mL flask. Then add 4-nitrophenyl tert-butoxyimide 5.0 g (20 mmol) and nitromethane 3.66 g (commercially available industrial product 98%, 60 mmol) successively under stirring, after stirring at room temperature for 2 hours, reduce The solvent was removed by rotary evaporation. The obtained crude product was directly separated by silica gel column chromatography (eluent: 1:3 diethyl ether / petroleum ether), and the product was dried in vacuum for 24 hours to obtain a light yellow product, which was 5.72 g of β-nitroamine derivatives, yield: 92 %, purity HPLC: 98.5%, optically pure HPLC, ee: 95%. Characterization data: nuclear magnetic resonance spectrum (hydrogen spectrum) 8.32 (d, 2H, J = 8.4 Hz), 7.55 (d, 2H, J = 8.4 Hz), 5.57 (brs, 1H), 5.46 (brs, 1H), 4.91 (m, 1H...
Embodiment 2
[0035] Example 2: Synthesis of (S)-1-nitro-2-(4-cyanophenyl)-2-tert-butoxyamide ethane
[0036]Add 0.49 g (1 mmol) of chiral copper complex (catalyst) and 40 mL of absolute ethanol as a solvent into a 100 mL flask. Then add 4.60 g (20 mmol) of 4-cyanophenyl tert-butoxyimide and 3.66 g of nitromethane (commercially available industrial product 98%, 60 mmol) successively under stirring, and after stirring at room temperature for 4 hours, reduce The solvent was removed by rotary evaporation. The obtained crude product was directly separated by silica gel column chromatography (eluent: 1:3 diethyl ether / petroleum ether), and the product was dried in vacuum for 24 hours to obtain a light yellow product, which was 5.35 g of β-nitroamine derivatives, yield: 92 %, purity HPLC: 98%, optically pure HPLC, ee: 93%. Characterization data: nuclear magnetic resonance spectrum (hydrogen spectrum) 7.70 (d, 2H, J = 8.0 Hz), 7.56 (d, 2H, J = 8.0 Hz), 5.56-5.53 (brs, 2H), 4.59 (m, 1H), 4...
Embodiment 3
[0037] Example 3: Synthesis of (S)-1-nitro-2-(3-pyridyl)-2-tert-butoxyamide ethane
[0038] Add 0.49 g (1 mmol) of chiral copper complex (catalyst) and 40 mL of absolute ethanol as a solvent into a 100 mL flask. Then add 4.12 g (20 mmol) of 3-tert-butoxyimide pyridine and 3.66 g (commercially available industrial products, 60 mmol) successively under stirring, stir and react at room temperature for 2 hours, and then rotary evaporate under reduced pressure Remove solvent. The obtained crude product was directly separated by silica gel column chromatography (eluent: 5% methanol / dichloromethane), and the product was dried in vacuum for 24 hours to obtain a colorless oily product, namely β-nitroamine derivative 5.23g, the yield: 98%, purity HPLC: 98%, optically pure HPLC, ee: 93%. Characterization data: NMR spectrum (H spectrum) 8.66-8.61 (brs, 2H), 7.80 (d, 1H, J = 8.0 Hz), 7.37 (m, 1H), 5.56-5.53 (dd, 2H, J = 9.2, 2.8 Hz), 4.65 (m, 1H), 4.54 (dd, 1H, J = 13.2, 2.8 Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com