Levofloxacin hydrochloride tablets
A technology of levofloxacin hydrochloride and tablets, which is applied in the direction of pill delivery, medical preparations of non-active ingredients, antibacterial drugs, etc., and can solve problems such as interfering with gastrointestinal absorption and not affecting the antibacterial activity of levofloxacin hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
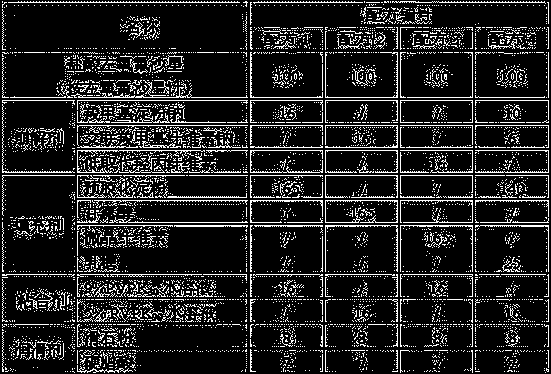
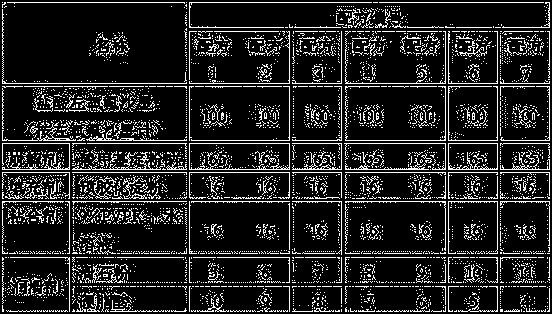
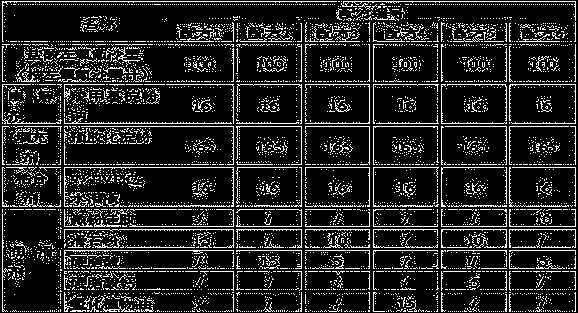
[0014] The present invention will be further described below by specific experimental scheme:
[0015] Evaluation indexes and detection methods such as hardness, compressibility, appearance in the experimental scheme of the present invention and the embodiment are as follows:
[0016] Hardness: Take the tablet and place it in a Monsanto hardness tester for testing, randomly select 20 tablets to measure the hardness respectively, and calculate the average value Y.
[0017] When Y is 30-50, it is recorded as "-", indicating that the hardness is good; when Y is lower than 30 or higher than 50, it is recorded as "+", indicating that the hardness is too low or too high.
[0018] Compressibility: Take the mixture of drug and excipients, put it in the tablet press machine, and observe the adhesion of the fine powder on the punch or die during the tablet compression process. "++" indicates that there is a lot of fine powder on the punch or die, and the sticking is serious; "+" indica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com