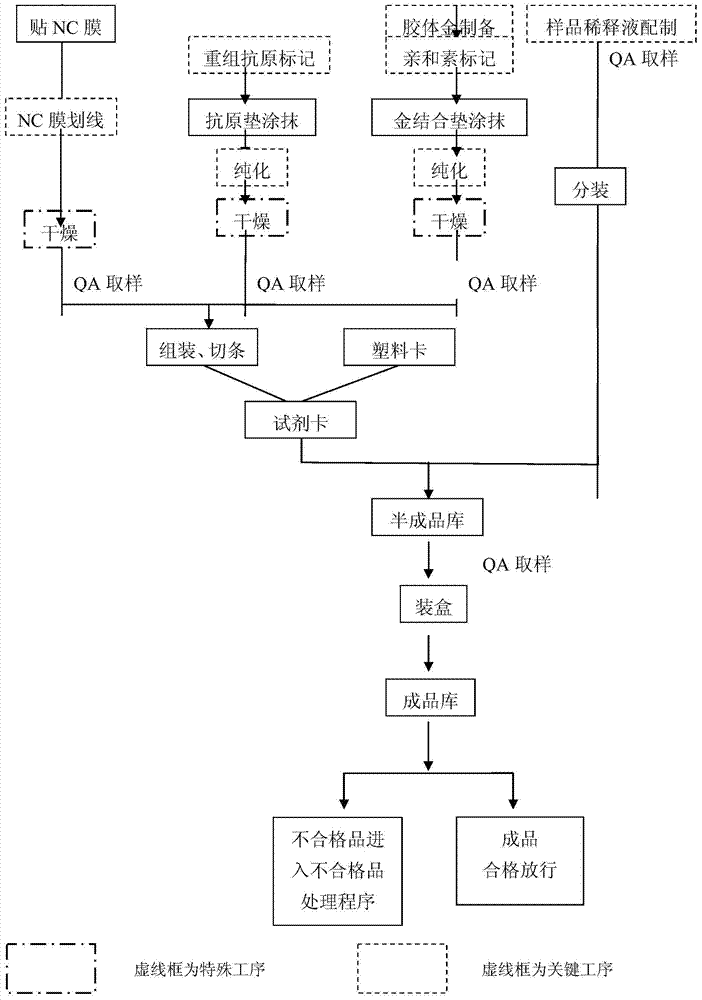
EB virus VCA-IgA antibody detection reagent and preparation method thereof
An Epstein-Barr virus and antibody detection technology, applied in measurement devices, disease diagnosis, instruments, etc., can solve the problems of competition interference, reduction of sensitivity and specificity of detection results, etc., to improve sensitivity and specificity, wide application range, and reduce operation effect of error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 detection reagent preparation condition screening
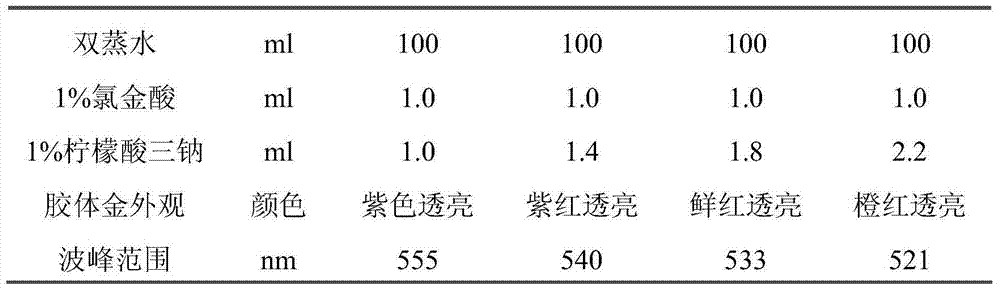
[0039] 1. Selection of colloidal gold particles
[0040] 1.1 Principle: Colloidal gold is prepared according to the redox reaction between chloroauric acid and trisodium citrate under boiling conditions. The particle size of the colloidal gold can be changed and controlled by adjusting the addition ratio of chloroauric acid and trisodium citrate.
[0041] 1.2 Preparation method
[0042] Add 1ml of 1% chloroauric acid solution to 100ml of double-distilled water and boil, add different amounts of 1% trisodium citrate solution under stirring conditions, continue to boil for 5 minutes to prepare colloidal gold particles of different sizes, and cool naturally for later use. Colloidal gold of different sizes was used to label avidin, and the company’s internal quality control product was used as the research material for detection. The results are shown in Table 1 and Table 2.
[0043] Table 1. Selection ex...
Embodiment 2
[0092] Embodiment 2. Reagent preparation
[0093] 1. Raw material requirements
[0094] 1.1 Recombinant Epstein-Barr virus VCA antigen
[0095] Manufacturer: Hong Kong Shennong Co., Ltd.
[0096] Product Name: Recombinant Epstein-Barr Virus VCA Antigen, Recombinant Epstein-Barr Virus NA1 Antigen
[0097] Batch number: 20131224E
[0098] Appearance: colorless transparent liquid;
[0099] Concentration and purity requirements: the concentration is greater than 2.0 mg / ml, determined by SDS-PAGA, and only one band exists under the condition of 10 microliters of sample.
[0100] 1.2 Mouse anti-human IgA antibody
[0101] Manufacturer: Hangzhou Longji Biotechnology Co., Ltd.
[0102] Product name: mouse anti-human IgA antibody
[0103] Batch number: 20131105
[0104] Appearance: colorless transparent liquid;
[0105] Concentration and purity requirements: the concentration is greater than 2.0 mg / ml, and it is determined by SDS-PAGA. The sample volume is 10 microliters and...
Embodiment 3
[0160] Embodiment 3.EB virus VCA-IgA antibody detection kit (colloidal gold method) preparation
[0161]Epstein-Barr virus VCA IgA antibody detection kit (colloidal gold method), including mouse anti-human IgA monoclonal antibody coated on the NC membrane detection line, biotin coated on the quality control line, and biotin-labeled antibody coated on the antigen pad Colloidal gold-labeled avidin coated on the recombinant Epstein-Barr virus VCA antigen and the gold label pad. Mouse anti-human IgA monoclonal antibody is a colorless transparent liquid with a concentration greater than 2.0mg / ml. It is determined by SDS-PAGA. There are two bands under the condition of 10μl of sample loading, and the coating concentration is 1.4mg / ml; avidin is Colorless transparent liquid, the concentration is greater than 2.0mg / ml, determined by SDS-PAGA, there is only one band under the condition of 10μl sample volume, the labeling concentration is 25μg / ml; biotin is a colorless transparent liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com