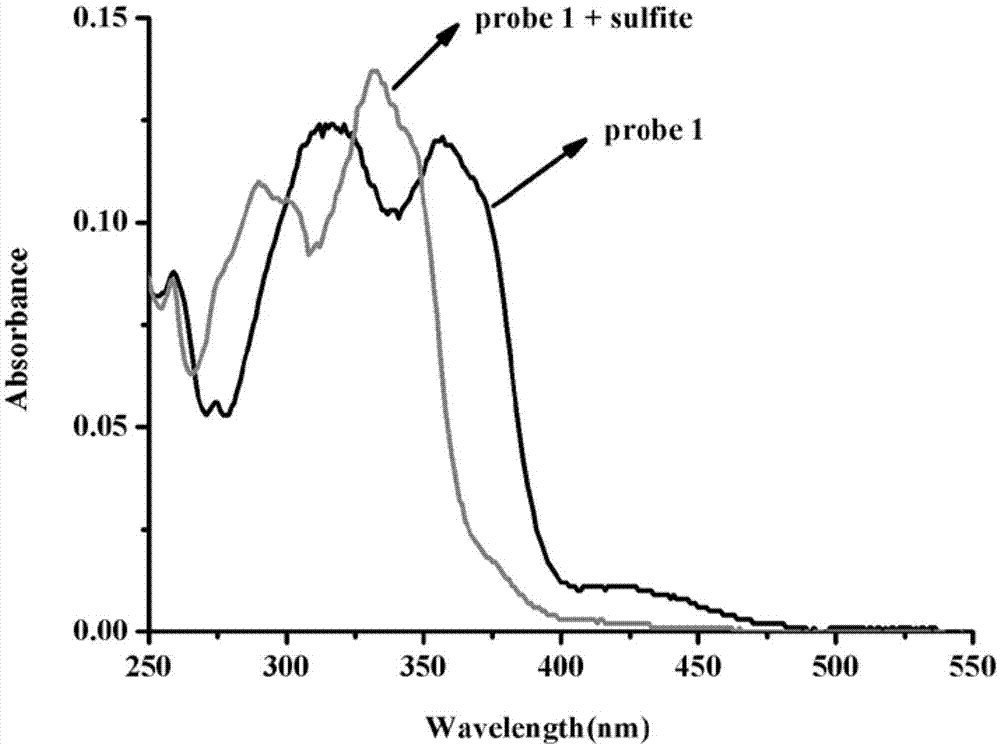
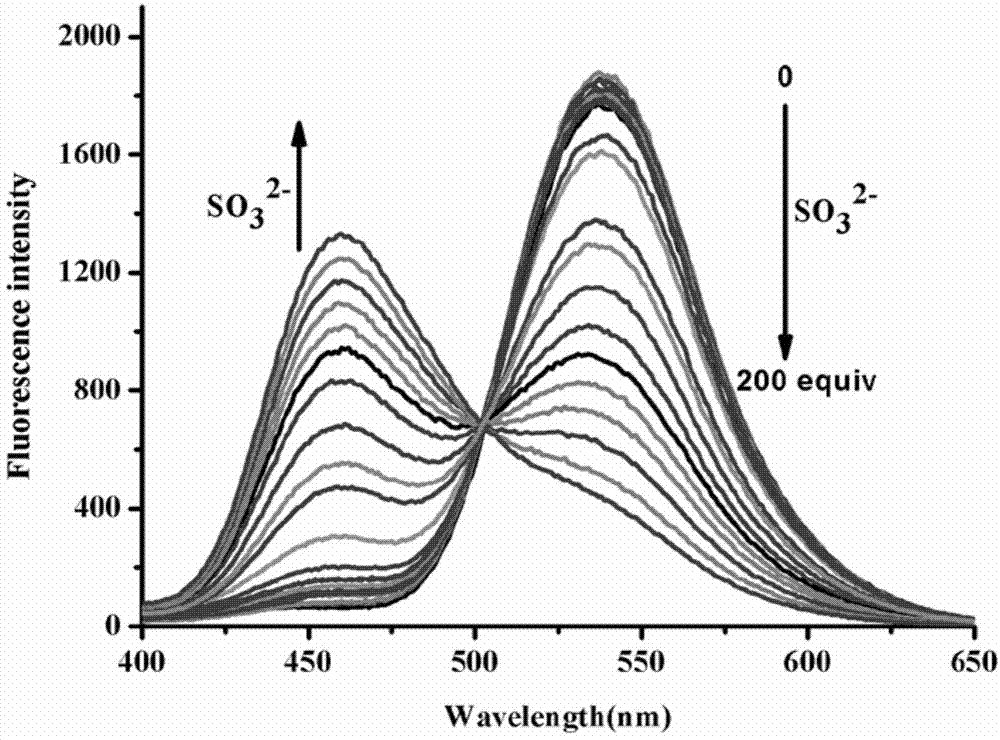
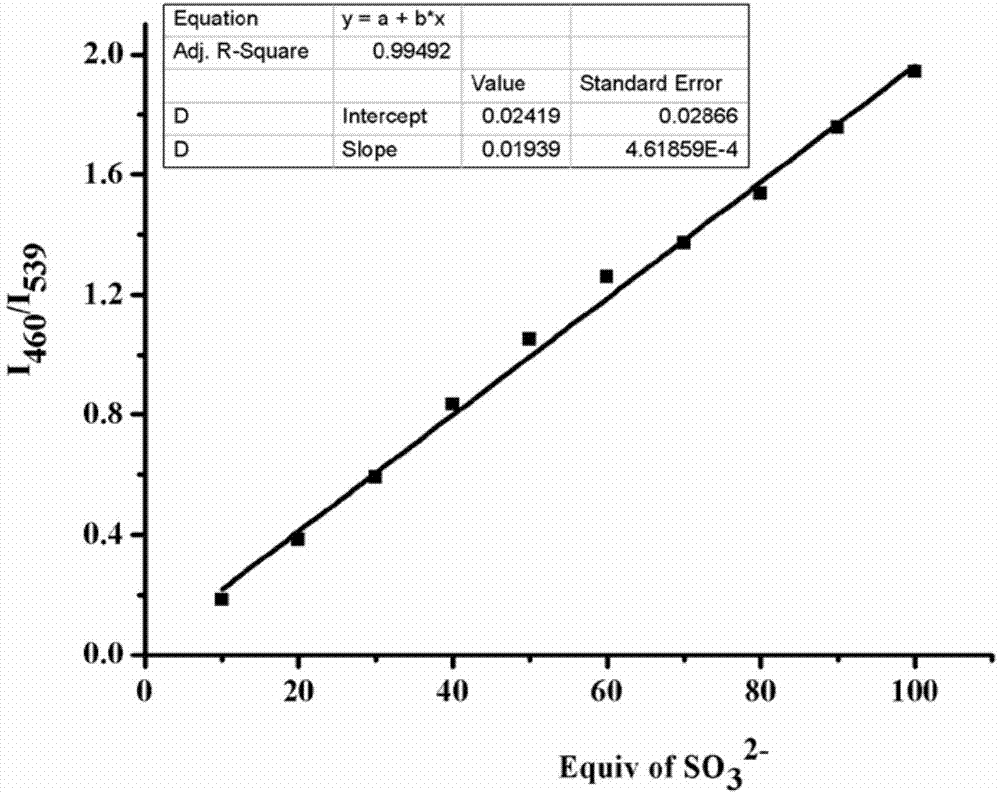
Preparation and application of sulfite ratiometric fluorescent probe
A ratio fluorescent probe, sulfite technology, applied in the field of chemical analysis and detection, to achieve the effect of simple synthesis, strong anti-interference ability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthesis of compound 1
[0020]Dissolve o-aminothiophenol (8.2 g, 66 mmol) and 4-methylsalicylic acid (10.0 g, 66 mmol) in 70 mL of toluene, heat to 55 °C under nitrogen protection, and stir for 2.5 h; then stop heating , naturally lowered the temperature to 35°C, slowly added 6.2 mL of phosphorus trichloride dropwise to the reaction solution, raised the temperature to 85°C and stirred for 5 h; the reaction was stopped, the solvent was removed by rotary evaporation, and the product 1 was obtained by column chromatography. Yield: 6.4 g. Yield: 40.3%. Compound 1 is characterized as follows: 1 H NMR (CDCl 3 ): δ = 12.46 (s, 1H), 7.97-7.95 (d, 1H), 7.89-7.87 (d, 1H), 7.58-7.56 (d, 1H), 7.51-7.47 (t, 1H), 7.41-7.37 (t, 1H), 6.92 (s, 1H), 6.78-6.76 (d, 1H), 2.37 (s, 3H).
Embodiment 2
[0021] Embodiment 2: the synthesis of compound 2
[0022] The product 1 (5.0 g, 20.7 mmol) and triethylamine (2.5 g, 24.9 mmol) obtained in the previous step were dissolved in 60 mL of dichloromethane, and acetyl chloride (1.95 g, 24.9 mmol) was slowly added dropwise thereto, and stirred at room temperature for 2 h; stop the reaction, add 40 mL of water to quench the reaction, extract with dichloromethane, wash with saturated brine, dry over anhydrous sodium sulfate, remove the solvent by rotary evaporation, and separate the product 2 by column chromatography. Yield: 4.7 g. Yield: 80%. Compound 2 is characterized as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 8.23 (d, J = 8.1 Hz, 1H), 8.11 (d, J = 8.1 Hz, 1H), 7.94 (d, J = 7.5 Hz, 1H), 7.53 (t, J = 7.7 Hz, 1H), 7.43 (m, 1H), 7.24 (d, J = 8.1, 1H), 7.09 (s, 1H), 2.51 (s, 3H), 2.46 (s, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 169.31, 162.75, 153.05, 148.12, 142.45, 135.21, 130.07, 127.43, 126.26, 125.20, 124.17, 123.38, 123.23, 12...
Embodiment 3
[0023] Embodiment 3: the synthesis of compound 3
[0024] The product 2 obtained in the previous step ( 3.5 g, 12.4 mmol), N-bromosuccinimide (2.4 g, 13.5 mmol), benzoyl peroxide (0.06 g, 0.025 mmol) were dissolved in 50 mL of dry carbon tetrachloride, refluxed for 24 h Stop the reaction, pour the reaction solution into ice water, extract with chloroform, dry over anhydrous sodium sulfate, remove the solvent by rotary evaporation, and separate the product 3 by column chromatography after vacuum drying. Yield: 2.3 g. Yield: 51.3%. Compound 3 is characterized as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 8.35 (d, J = 8.1 Hz, 1H), 8.12 (d, J = 7.9 Hz, 1H), 7.96 (d, J = 7.4 Hz, 1H), 7.58 – 7.52 (m, 1H), 7.48 – 7.42 (m, 2H), 7.33 (s, 1H), 4.54 (s, 2H), 2.52 (s, 3H). 13 C NMR (126 MHz, CDCl 3 ) δ 168.97, 161.79, 152.91, 148.23, 141.31, 135.35, 130.68, 127.00, 126.46, 126.03, 125.56, 124.31, 123.47, 121.39, 31.73, 21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com