Brush-type difluoro monomer and synthetic method thereof
A synthesis method and technology of fluorine monomers, applied in the field of materials, can solve the problems of difficult synthesis, few types of side chain monomers, and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Taking the preparation of a brush-type difluoromonomer with the following structural formula as an example, the specific preparation method is as follows:
[0032]
[0033] 1. Preparation of compound a
[0034] Under the protection of nitrogen, add 0.79g (32.42mmol) of magnesium powder and 5mL of dry tetrahydrofuran to a 100mL three-necked flask, turn on the magnetic stirring, raise the temperature to 65°C, and slowly add 5.00g (27.02mmol) of 3,5-dimethyl A mixed solution of bromobenzene and 40 mL of dry tetrahydrofuran was stirred at 65°C for 3 hours to prepare a Grignard reagent, and then a mixed solution of 3.76 g (27.02 mmol) of 2,6-difluorobenzonitrile and 40 mL of dry tetrahydrofuran was slowly added dropwise, Continue to stir at 65°C for 6 hours. After the reaction is over, add 80mL of 0.1mol / L hydrochloric acid aqueous solution, stir and reflux at 80°C for 12 hours, wash the reaction solution until neutral, concentrate the organic phase, and prepare compound ...
Embodiment 2
[0042] Taking the preparation of a brush-type difluoromonomer with the following structural formula as an example, the specific preparation method is as follows:
[0043]
[0044] In step 3 of Example 1, the 3,5-dimethylphenylboronic acid used was replaced with equimolar 4-methylbenzeneboronic acid, and the other steps were the same as in Example 1 to prepare a brush-type difluoromonomer, which The yield is 60.1%, and the structural characterization data are as follows:
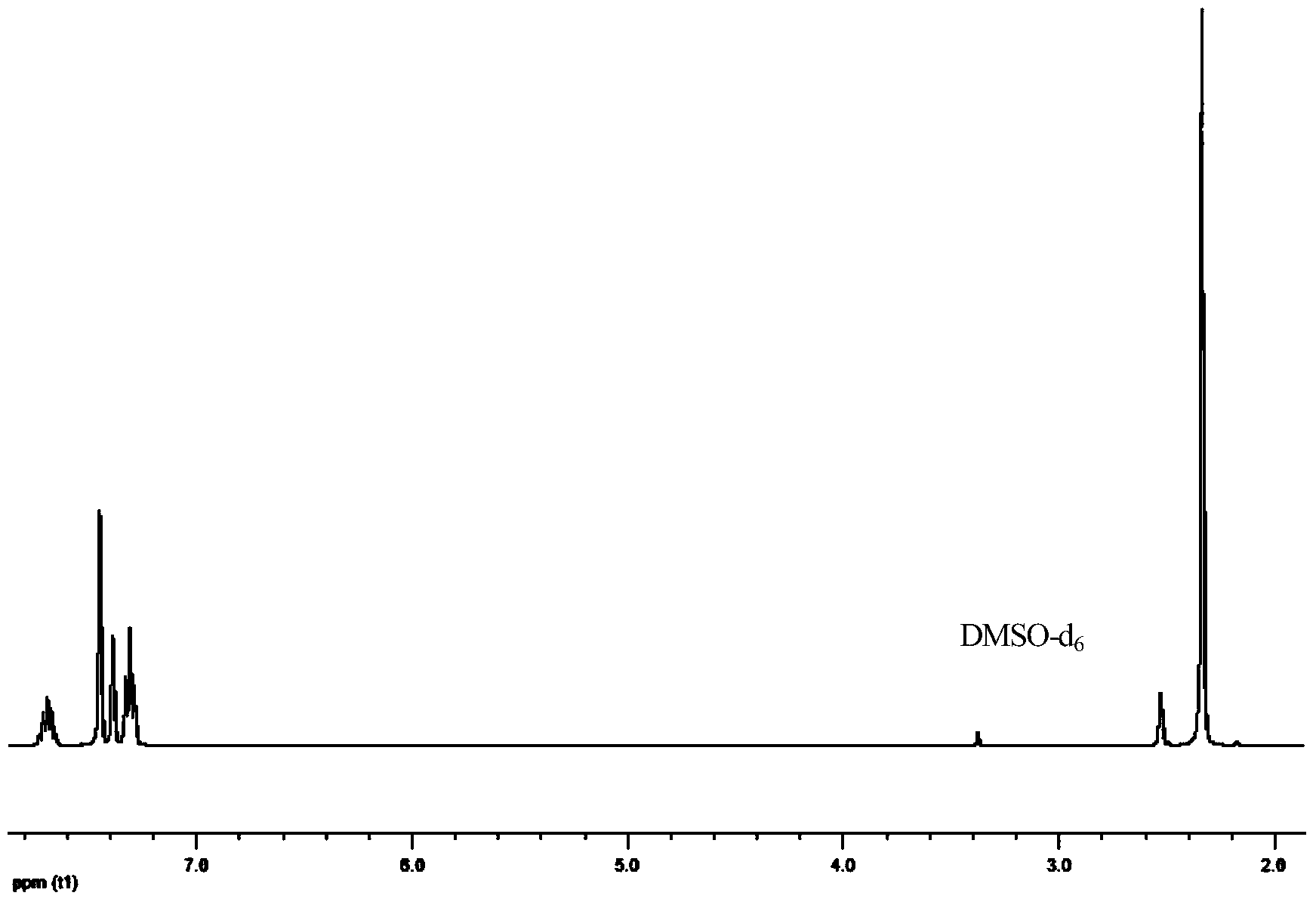
[0045] 1 H-NMR (DMSO-d 6 as solvent, TMS as internal standard, 300MHz, ppm): 7.66(m, 1H), 7.48(s, 3H), 7.26(t, 2H), 7.11(s, 8H), 3.89(s, 4H), 2.19( s, 6H).
Embodiment 3
[0047] Taking the preparation of a brush-type difluoromonomer with the following structural formula as an example, the specific preparation method is as follows:
[0048]
[0049] In step 3 of Example 1, the 3,5-dimethylphenylboronic acid used was replaced with equimolar 2,4-dimethylphenylboronic acid, and the other steps were the same as in Example 1 to prepare a brush-type difluoromono body, its yield is 56.9%, and the structural characterization data are as follows:
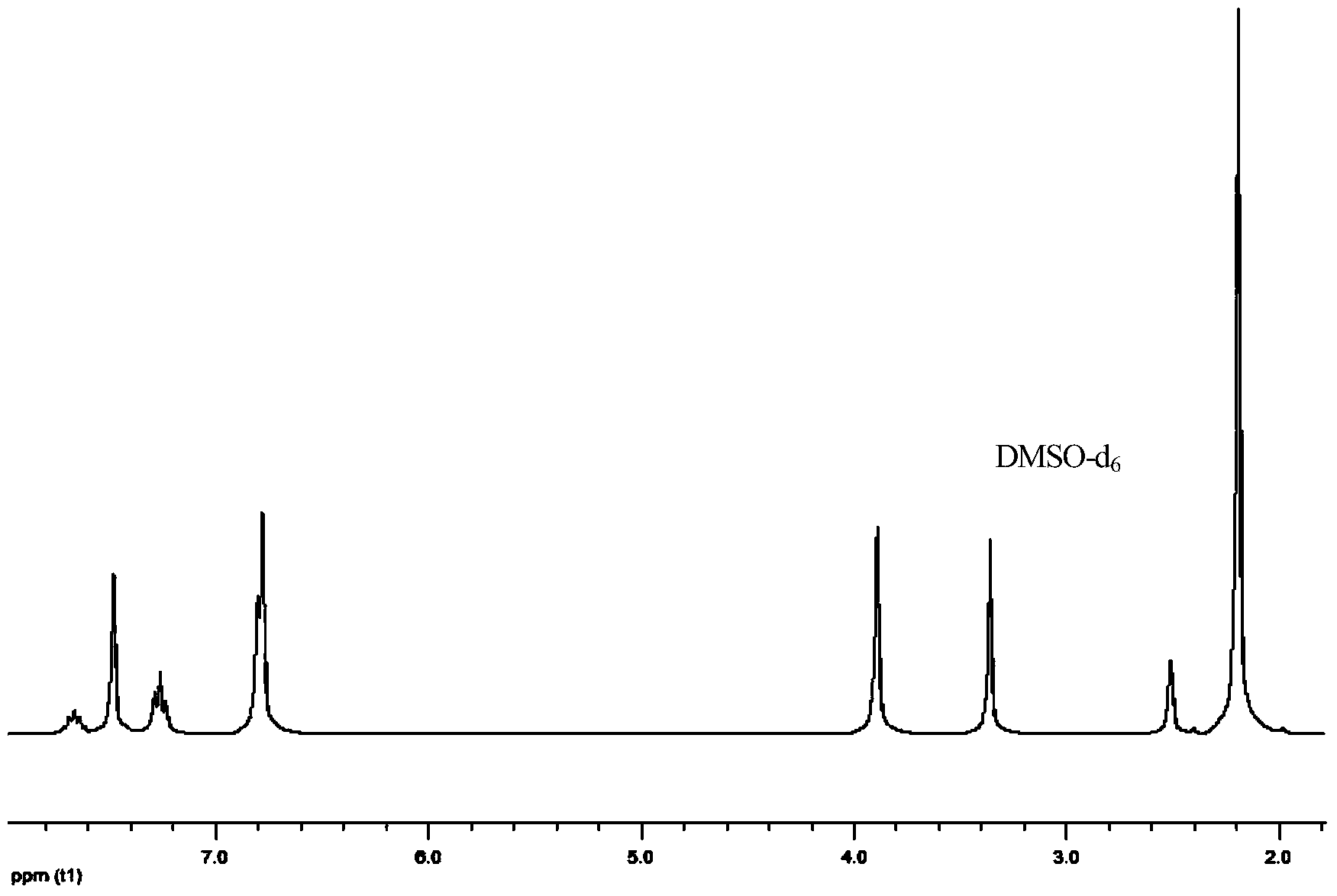
[0050] 1 H-NMR (DMSO-d 6 as solvent, TMS as internal standard, 300MHz, ppm): 7.56(m, 1H), 7.41(s, 3H), 7.22(t, 2H), 7.12(s, 2H), 6.99(s, 2H), 6.98( s, 2H), 3.96 (s, 4H), 2.34 (s, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com