Streptococcus suis vaccine composition, and preparation method and application thereof
A vaccine composition, Streptococcus suis technology, applied in the field of veterinary biological products, can solve the problems of poor immunogenicity of capsular polysaccharides and unsatisfactory results, no vaccine Streptococcus suis infection, limitations of immune protection, etc. , to achieve the effect of preventing and/or treating Streptococcus suis infection, perfecting Streptococcus suis infection, and facilitating large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Immunogenic Antigen of Streptococcus suis
[0046] S. suis immunogenic antigens can be prepared by various methods known to those skilled in the art, such as by cloning or expression vectors comprising polynucleotides of the present invention. . Publicly available vectors suitable for preparing S. suis polypeptide antigens of the invention include plasmids, adenoviruses, baculoviruses, yeast baculoviruses, plant viruses, adeno-associated viruses, retroviruses, herpes simplex virus, alphaviruses, lentiviruses, Viruses, etc., and methods for constructing such vectors are also available. The preparation of the Streptococcus suis polypeptide antigen in the invention also includes artificially synthesized polypeptide antigens. The embodiment of the present invention adopts E. coli expression system to prepare 38KDA protein and SaoA protein.
[0047] 1. Materials
[0048] The pMD18-T vector used in the present invention (purchased from Treasure Bioengineeri...
Embodiment 2
[0068] Preparation of Streptococcus suis vaccine composition
[0069] Take the 38KDA and SaoA proteins prepared in Example 1 and slowly add them to the adjuvant. During the process of adding, stir continuously for 12 minutes with an emulsifier with a rotation speed of 800 rpm, mix well, and store at 4°C. This is the vaccine composition for Streptococcus suis. See Table 2 for specific ratios. Adjuvants suitable for the present invention can be oil adjuvants well known to those skilled in the art, aqueous emulsions (for example, complete Freund's adjuvant and incomplete Freund's adjuvant), water-soluble adjuvants, Quil A adjuvant, hydrogen One or more of aluminum oxide adjuvant, dextran, dextran sulfate, and sodium alginate. In this embodiment, the water-soluble adjuvant gel adjuvant (Sepic company, France) is selected.
[0070] Table 2 Streptococcus suis vaccine composition ratio
[0071]
Embodiment 3
[0073] Immunogenicity test of Streptococcus suis vaccine composition
[0074] 1. Immunization procedure
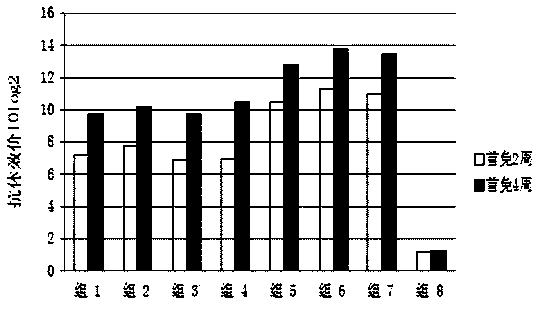
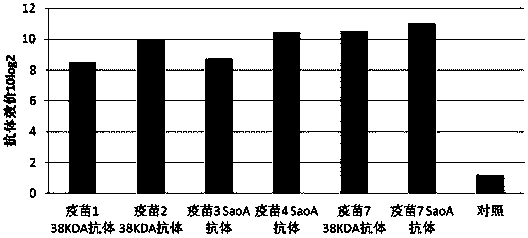
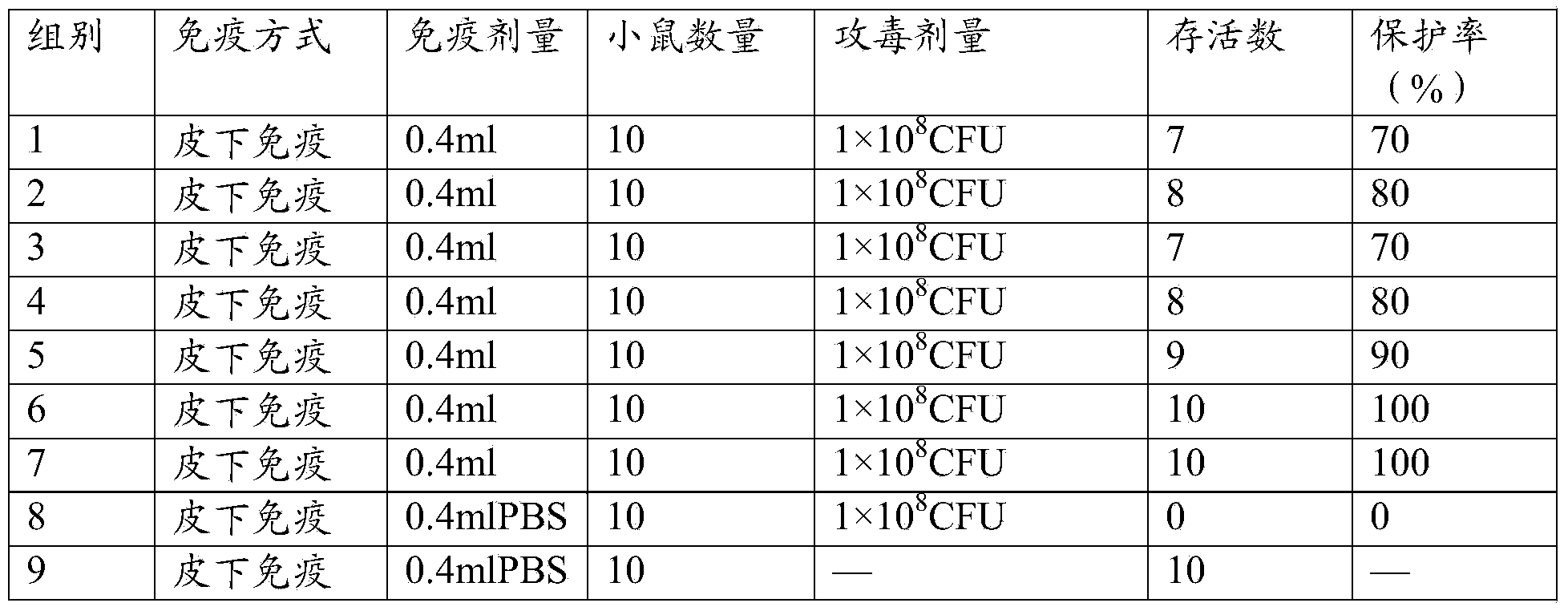
[0075] Use 5-7 week-old clean level Kunming mice for immunogenicity test, divide into 8 groups according to test requirements, 1-7 groups are respectively vaccine 1, vaccine 2, vaccine 3, vaccine prepared by Example 2 of the present invention 4. Vaccine 5, vaccine 6, vaccine 7 immunization groups, group 8 PBS control group. The immune group was immunized by subcutaneous injection of 0.4ml on the back, and the PBS control group was immunized with the same amount of PBS, followed by booster immunization once 14 days later. The antibody levels of vaccine 1, vaccine 2, vaccine 3, vaccine 4, vaccine 5, vaccine 6, vaccine 7 and the PBS control group were detected 14 days and 28 days after the first immunization, respectively.
[0076] 2. Detection of antibody level
[0077] The mice in each group were blood collected 14 days and 28 days after the first immunization, and the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com