Method of detecting impurities in penehyclidine hydrochloride
A penehyclidine hydrochloride and detection method technology, applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of large operation error, inconspicuous color development, low detection sensitivity, etc., so as to improve the detection sensitivity and stabilize the analysis method. , the effect of increasing controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Main main instruments and chromatographic conditions:
[0044] TRACE GC ULTRA gas chromatograph, equipped with hydrogen flame ionization detector (FID), temperature 300 ℃, automatic sampler, Chromeleon chromatographic workstation.
[0045] Chromatographic column: Rtx?-5 Amine (30m×0.25mm, 0.5μm)
[0046] Column temperature: the initial temperature is 90°C, keep for 1 minute, then raise the temperature to 300°C at a rate of 8°C per minute, and keep for 8 minutes.
[0047] Column flow rate: 2. 5ml / min;
[0048] Inlet temperature: 190°C;
[0049] Split ratio: 4:1;
[0050] Injection volume: 2ul.
[0051] Penehyclidine hydrochloride was provided by Chongqing Xianyang Pharmaceutical Technology Co., Ltd., batch number: 110900.
[0052] The impurity II reference substance was provided by Chongqing Xianyang Pharmaceutical Technology Co., Ltd., batch number 110601.
[0053] The impurity III reference substance was purchased from Sigma, batch number MKBF3377V.
[0054] Pre...
Embodiment 2
[0060] According to the above chromatographic conditions and operation methods, the specificity, precision, linearity, detection limit and quantification limit, sample recovery rate and stability of the method were respectively verified by methodology, and the results are as follows:
[0061] (1) Specificity:
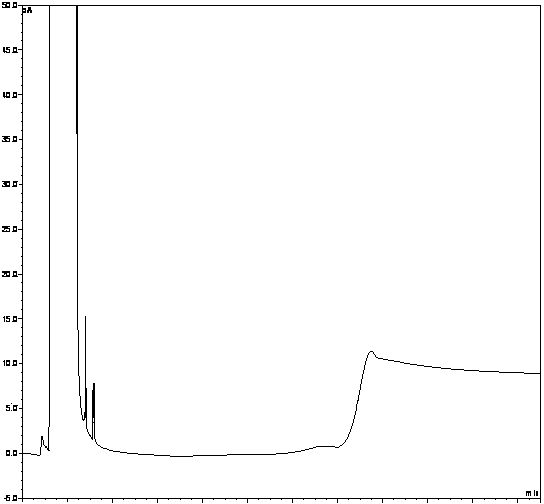
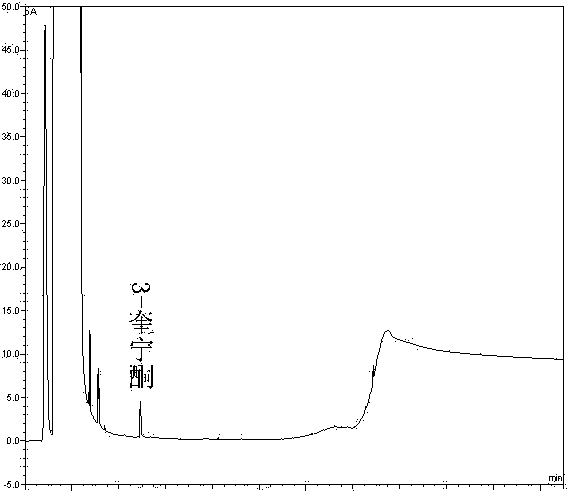
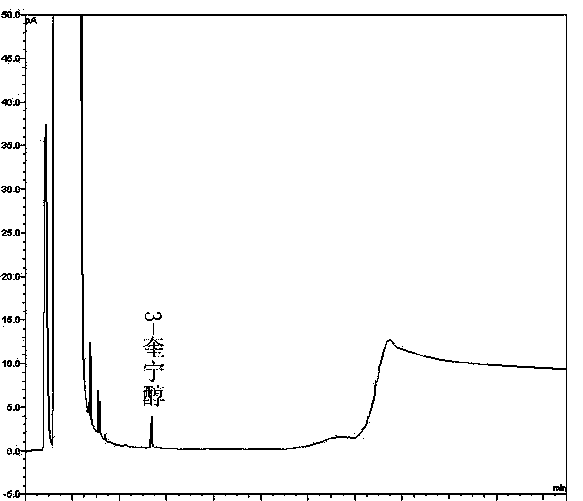
[0062] Take the solvent DMF, the solution of the test product, the solution of the impurity reference substance II, the solution of the impurity reference substance III, the mixed solution of the test product solution and the solutions of the impurity reference substance II and III, and inject the samples in sequence. The results show that the solvent has peaks with retention times of 1.370min, 2.565min, 2.939min, and 3.331min, the retention time of impurity II is 5.113min, the retention time of impurity III is 5.557min, and the retention time of penehyclidine is 14.948min, indicating that the solvent The separation degree from the impurity reference substance i...
Embodiment 3
[0095] With reference to the method of Example 1, the temperature of the injection port is investigated. The results are shown in Table 6:
[0096] Table 6 Inspection results of injection port temperature
[0097]
[0098] The above results show that when the temperature of the injection port is measured in the range of 150-230°C, the impurities II and III are well separated and accurate determination can be realized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com