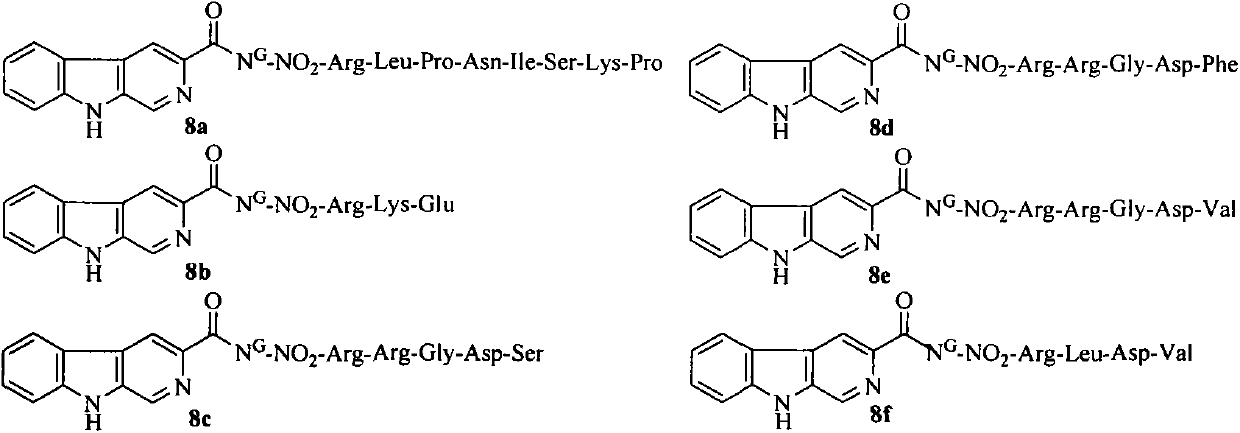
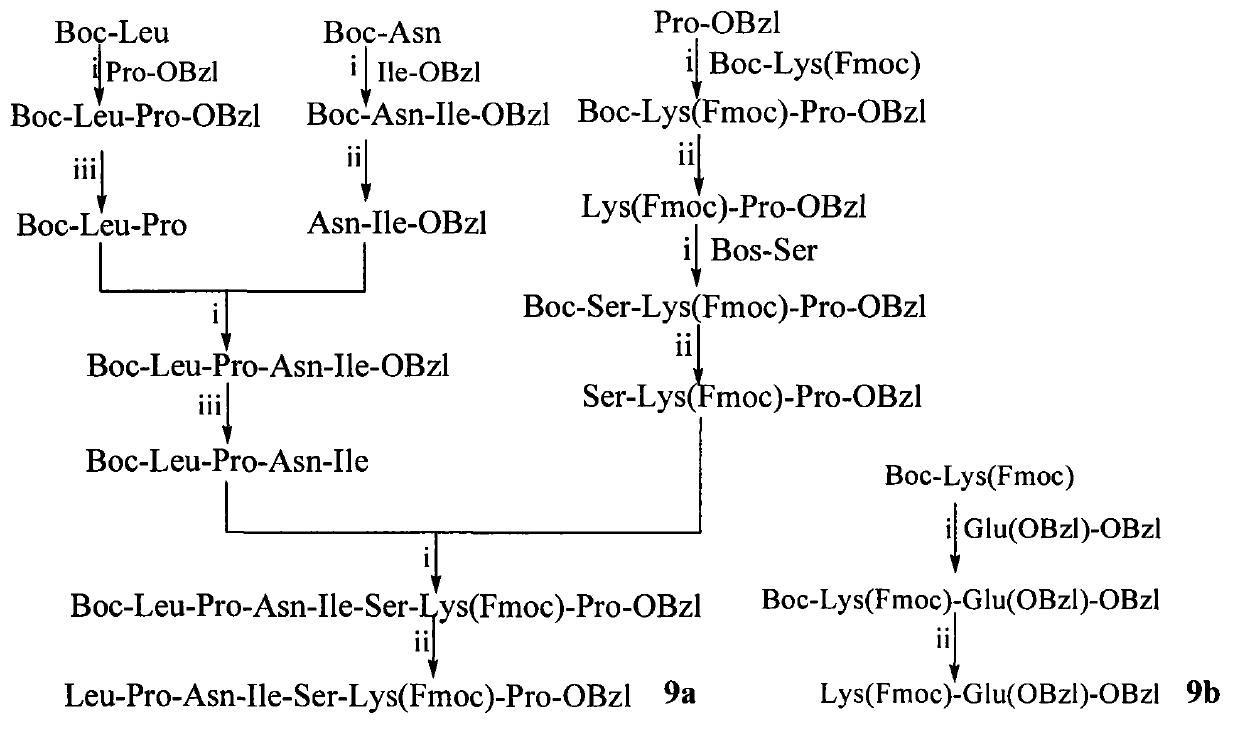
Conjugates of beta-carboline-3-carboxylic acid and oligopeptides, preparation, nano structure, and application thereof as antitumor agent
A conjugate, arg-gly-asp-val technology, is applied in the field of conjugation of β-carboline-3-carboxylic acid and 6 kinds of oligopeptides, which can solve the problems of toxic reaction and inhibition of tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 prepares 3S-1,2,3,4-tetrahydro-β-carboline 3-carboxylic acid (1)
[0027] Slowly drop 0.1 mL of concentrated sulfuric acid into 200 mL of distilled water with stirring at room temperature. Add 2.520 g (12.4 mmol) of L-tryptophan to the obtained dilute sulfuric acid solution and ultrasonically shake until the L-tryptophan is completely dissolved. To the resulting solution was added 5 mL of 37% formaldehyde solution. The resulting colorless solution was stirred at room temperature for 6 hours, monitored by thin layer chromatography (dichloromethane:methanol, 3:1) until L-tryptophan disappeared, and the reaction was terminated. Concentrated ammonia water was slowly added dropwise to the reaction solution, adjusted to pH 6, and left to stand for half an hour. Buchner funnel decompressed to filter out the generated precipitate, washed the precipitate with water and acetone repeatedly 3 times, spread the filtered colorless solid on a petri dish, placed it in a ...
Embodiment 2
[0028] Example 2 Preparation of 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (2)
[0029] Slowly add 2.6 mL of thionyl chloride dropwise into 25 mL of methanol under ice-cooling and stirring. After activation for 30 minutes, add 2.16 g (10 mmol) of 3S-1,2,3,4-tetrahydro-β-carboline-3 -Carboxylic acid (1), the resulting colorless solution was stirred at room temperature for 18 hours, monitored by thin layer chromatography (dichloromethane:methanol, 2:1) until the starting point disappeared, and the reaction was terminated. The reaction mixture was concentrated to dryness under reduced pressure, the residue was dissolved in 30 mL of methanol, and the resulting solution was concentrated to dryness under reduced pressure. This operation was repeated once more. The residue was dissolved in 30 mL of ether, and the resulting solution was concentrated to dryness under reduced pressure. This operation was repeated once more. The residue was purified by column chr...
Embodiment 3
[0030] Embodiment 3 prepares β-carboline-3-carboxylic acid methyl ester (3)
[0031] Add 2.0g (9.26mmol) 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester to 100mL acetone under ice bath and stirring, slowly add 3.1g (13.89mmol) Potassium manganate powder. The reaction mixture was stirred at room temperature for 12 h, monitored by thin layer chromatography (dichloromethane:methanol, 10:1) until the disappearance of 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylate methyl ester, terminated reaction. Add 100 mL of ethanol and stir for 30 minutes to quench the unreacted potassium permanganate, remove manganese dioxide by filtration under reduced pressure, and concentrate the filtrate to dryness under reduced pressure to obtain a light yellow oil. The resulting mixture was separated by column chromatography (dichloromethane:methanol, 25:1) to afford 1.05 g (54%) of the title compound as a colorless powder. ESI-MS(m / e): 228[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com