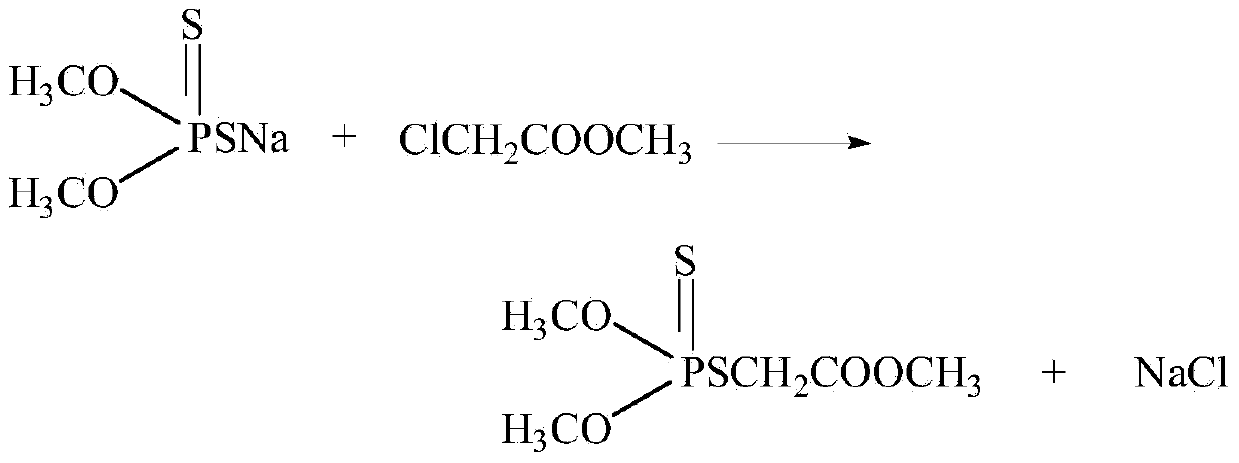
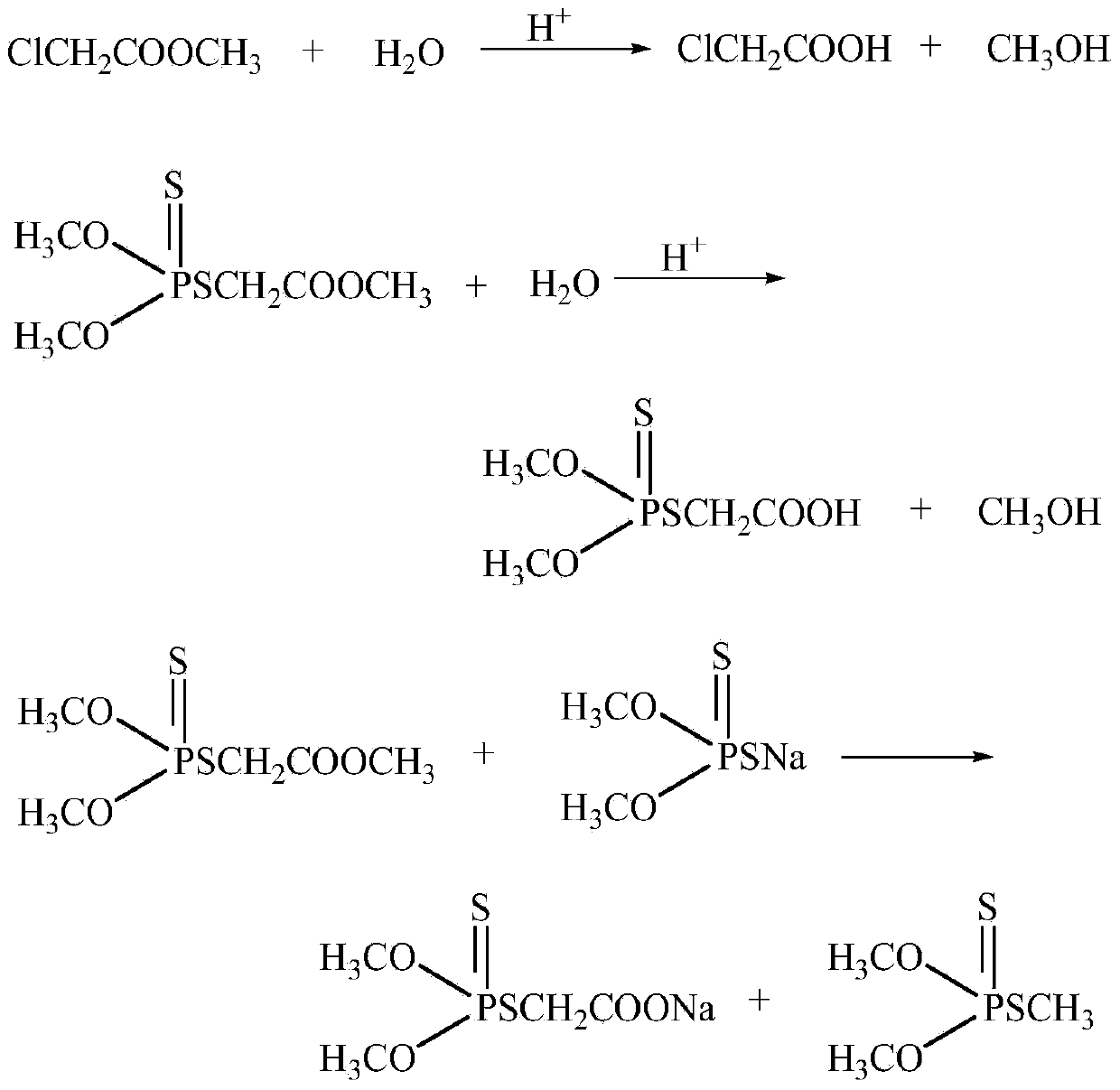
Synthetic method for thiophosphate
A synthetic method, a technology of phosphorothioate, which is applied in the field of intermediate preparation and can solve problems such as low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 100g of ammonium thiophosphate salt with a mass fraction of 52%, was added with 49.3g of methyl chloroacetate at a molar ratio of 1:1.5, and the reaction temperature was controlled at 60°C. After 2.0 hours of reaction, 80.3g of the oil phase and 68g of the water phase were separated. The ammonium sulfate phosphate content in the waste water is 3.8%. The oil phase was vacuum distilled to separate methyl chloroacetate, and the mass fraction of phosphorothioate was 89.8%, and the yield of phosphorothioate was 90.4%.
Embodiment 2
[0033] 100g of sodium thiophosphate with a mass fraction of 50%, 61.5g of methyl chloroacetate was added at a molar ratio of 1:2.0, the reaction temperature was controlled at 58°C, and the reaction was carried out for 2.5 hours to separate 90.2g of the oil phase and 70.3g of the water phase , the sodium phosphoric acid sulfur content in the waste water is 2.9%. The oil phase was vacuum distilled to separate methyl chloroacetate to obtain a mass fraction of phosphorothioate of 90.1%, and a yield of phosphorothioate of 91.3%.
Embodiment 3
[0035] Get 68g of the water phase after the reaction in Example 1, add 49.3g of methyl chloroacetate in the first step, control the reaction temperature to be 60°C, and react for 60 minutes to separate 51.5g of the methyl chloroacetate phase, and the sulfur in the waste water The content of ammonium phosphate is 1.1%, and it will be discharged after wastewater treatment. In the second step, 51.5 g of the methyl chloroacetate phase obtained in the first step is added to 100 g of 52% ammonium sulfuric acid phosphate, the controlled reaction temperature is 58 ° C, and the reaction time is 80 minutes. 82.4g of the oil phase, and the water phase is recycled for the next reaction. The oil phase is distilled at a temperature lower than 115°C and the vacuum degree is higher than 0.095Mpa to separate methyl chloroacetate by distillation to obtain phosphorothioate. The reaction was repeated ten times to obtain an average mass fraction of phosphorothioate of 90.4%, and an average yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com