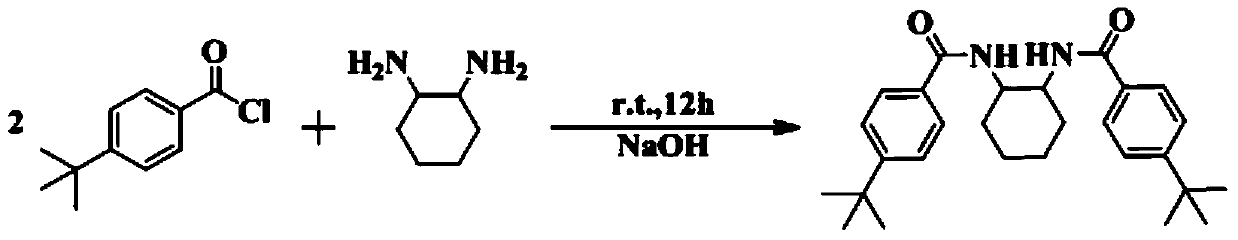Bridged bisamido rare-earth amide compounds, and preparation method and application thereof
A bridging bisamide-based rare earth technology, which is applied in the field of catalysts and their preparation, can solve the problems of poor catalyst regioselectivity, limited catalyst metal types, harsh reaction conditions, etc., and achieve short reaction time, high yield, and easy access to raw materials. The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
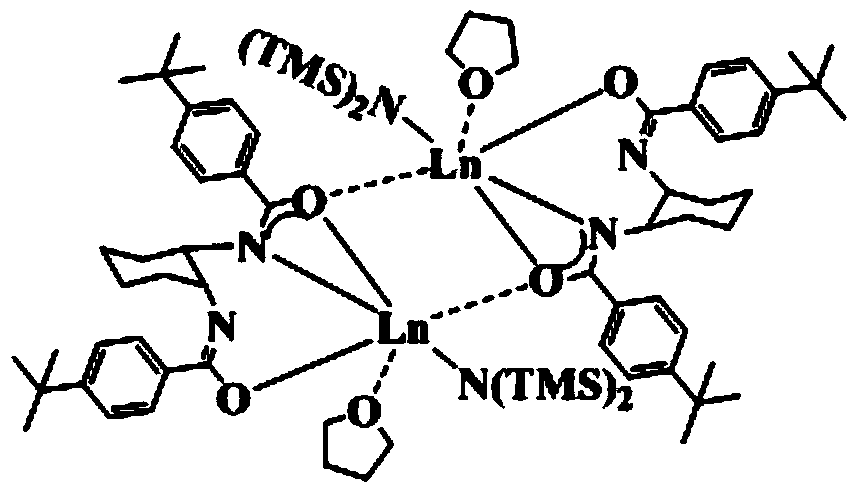
[0046] Embodiment 1: prepare {LLa[N(SiMe 3 ) 2 ]·THF} 2
[0047] 1) Before preparing the bridged bisamide-based rare earth amides, prepare LH 2 , its preparation method is as follows:
[0048] Synthesis of N,N'-(cyclohexane-1,2-diyl)bis(4-tert-butylbenzamide): weigh a certain molar amount of cyclohexanediamine in a round bottom flask, and measure it at 1.6ml / mmol Dilute sodium hydroxide solution in a round-bottomed flask, then weigh p-tert-butylbenzoyl chloride at a molar ratio of 2:1, slowly drop p-tert-butylbenzoyl chloride into the round-bottomed flask, and react at room temperature After 6 hours, the reaction stopped. Suction filtration with a Buchner funnel, the solid was washed with deionized water until neutral, dried under an infrared lamp, and then recrystallized with anhydrous methanol to obtain compound LH 2 , yield 80%. NMR data: 1 H NMR (400MHz, CDCl 3 ): δ7.71(d, J=8.6Hz, 4H, ArH); 7.39(d, J=8.6Hz, 4H, ArH); 7.00(d, J=6.4Hz, 2H, NH); 3.96(s, 2H, CH); 2....
Embodiment 2
[0053] Embodiment 2: preparation {LNd [N (SiMe 3 ) 2 ]·THF} 2
[0054] Steps (1), (2) and (3) of present embodiment 2 are the same as embodiment 1, wherein the reaction time in step (1) is 9 hours, in step (2) in a dehydration deoxygenation, argon protection Add Nd[N(SiMe 3 ) 2 ] 3 , the ether solvent was changed to diethyl ether, and the reaction time was 20 hours; after the reaction was completed, after post-treatment, the room temperature was left to stand until crystals were precipitated, which was the bridged bisamide-based rare earth aminate {LNd[N(SiMe 3 ) 2 ]·THF} 2 .
[0055] {LNd[N(SiMe 3 ) 2 ]·THF} 2 Data: 88% yield. Elemental analysis: C, 56.40; H, 7.98; N, 5.23; Nd, 17.85. C 76 h 126 N 6 o 6 Nd 2 Si 4 Theoretical: C, 56.32; H, 7.84; N, 5.19; Nd, 17.80. Infrared absorption spectrum data (KBr, cm -1 ): 3439(s), 2963(w), 2359(m), 1635(m), 1541(s), 1384(s), 1240(s), 1216(s), 1156(vs), 504(s ).
Embodiment 3
[0056] Embodiment 3: preparation {LSm [N (SiMe 3 ) 2 ]·THF} 2
[0057] Steps (1), (2) and (3) of present embodiment 3 are the same as embodiment 1, wherein the reaction time in step (1) is 12 hours, in step (2) in a dehydration deoxygenation, inert gas protection Sm[N(SiMe 3 ) 2 ] 3 , the ether solvent is replaced by ethylene glycol dimethyl ether, and the reaction time is 24 hours; after the reaction is completed, after the post-treatment, it is allowed to stand at room temperature until crystals are precipitated, which is the bridged bisamide-based rare earth aminate {LSm[N(SiMe 3 ) 2 ]·THF} 2 .
[0058] {LSm[N(SiMe 3 ) 2 ]·THF} 2 Data: Yield 78%. Elemental analysis: C, 56.05; H, 7.85; N, 5.20; Sm, 18.48. C 76 h 126 N 6 o 6 SM 2 Si 4 Theoretical: C, 55.90; H, 7.78; N, 5.15; Sm, 18.42. Infrared absorption spectrum data (KBr, cm -1 ): 3440(s), 2963(w), 2361(m), 2337(m), 1634(m), 1541(s), 1507(w), 1384(s), 1236(s), 1219(s ), 1157(vs), 555(m), 505(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com