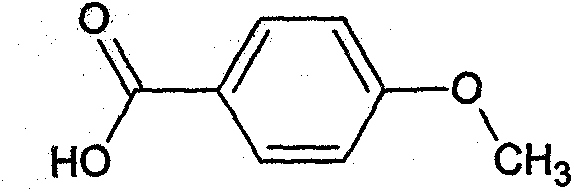
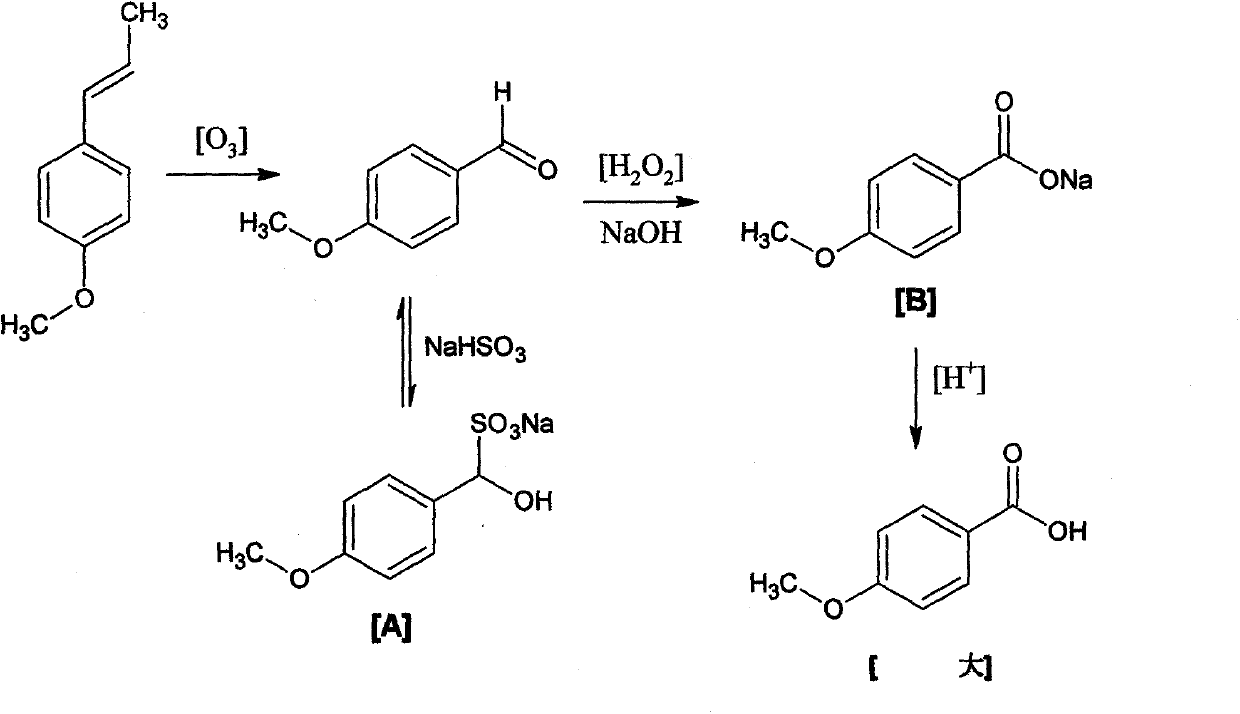
Preparation method of anisic acid
A technology of anisic acid and anisole, which is applied in the field of preparation of anisic acid, can solve the problems of application limitation, heavy metal content and organic solvent residue exceeding the standard, and achieves the effects of easy operation, danger prevention and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] S1, take an appropriate amount of crude sulfate turpentine and carry out fractional distillation to obtain crude anethole;
[0027] S2. Crystallizing and separating the crude anethole obtained in step S1 to obtain high-purity anethole;
[0028] S3, high-purity anethole, NaHSO 3 Put the solution into a reaction kettle, pass through ozone, and oxidize for 110 minutes to obtain anisaldehyde sodium sulfite adduct A;
[0029] S4. Slowly add sodium hydroxide solution and 30% hydrogen peroxide to A simultaneously, stir and keep at 65° C. for 5.0 h to obtain reaction product B;
[0030] S5. After the reaction is terminated, add HCl solution to B to adjust the pH value to 1, and filter to obtain anisic acid.
[0031] NaHSO in the step S3 3 The volume ratio of high-purity anethole is 1.2:1.
[0032] It is determined through experiments that the purity of the anethole obtained in step S2 is above 99.5%, the product yield of step S3 is ≥84%, and the purity is ≥98%.
Embodiment 2
[0034] S1, take an appropriate amount of crude sulfate turpentine and carry out fractional distillation to obtain crude anethole;
[0035] S2. Crystallizing and separating the crude anethole obtained in step S1 to obtain high-purity anethole;
[0036] S3, high-purity anethole, NaHSO 3 Put the solution into a reaction kettle, pass through ozone, and oxidize for 110 minutes to obtain anisaldehyde sodium sulfite adduct A;
[0037] S4. Slowly add sodium hydroxide solution and 30% hydrogen peroxide dropwise to A at the same time, stir and keep at 85° C. for 9.0 h to obtain reaction product B;
[0038] S5. After the reaction is terminated, add HCl solution to B to adjust the pH value to 3, and filter to obtain anisic acid.
[0039] Wherein, in the step S2, NaHSO 3 The volume ratio of high-purity anethole is 1.2:1.
[0040] It is determined through experiments that the purity of the anethole obtained in step S2 is above 99.5%, the product yield of step S3 is ≥84%, and the purity ...
Embodiment 3
[0042] A preparation method for anisic acid, comprising the steps of:
[0043] S1, take an appropriate amount of crude sulfate turpentine and carry out fractional distillation to obtain crude anethole;
[0044] S2. Crystallizing and separating the crude anethole obtained in step S1 to obtain high-purity anethole;
[0045] S3, high-purity anethole, NaHSO 3 Put the solution into a reaction kettle, pass through ozone, and oxidize for 110 minutes to obtain anisaldehyde sodium sulfite adduct A;
[0046] S4. Slowly add sodium hydroxide solution and 30% hydrogen peroxide dropwise to A at the same time, stir and keep at 75° C. for 7.0 h to obtain reaction product B;
[0047] S5. After the reaction is terminated, add HCl solution to B to adjust the pH value to 2, and filter to obtain anisic acid.
[0048] Wherein, in the step S3, NaHSO 3 The volume ratio of high-purity anethole is 1.2:1.
[0049]It is determined through experiments that the purity of the anethole obtained in step ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com