Method for improving structure of ACE inhibitory peptide prepared from food protein
A technology for inhibiting peptides and foods, applied in the field of biotechnology research, can solve problems such as poor absorption, easy degradation, and weak biological effects, and achieve the effects of improving biological activity, improving bioavailability, and enhancing biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
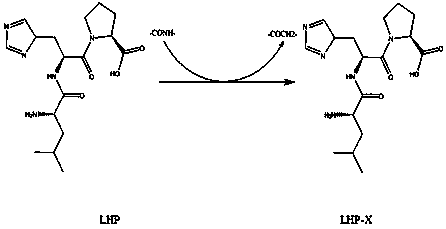
[0018] (1) Leu-His-Pro (codenamed LHP) is a tripeptide with strong angiotensin-converting enzyme inhibitory activity. In vitro inhibitory activity IC of LHP on ACE 50 was 1.6 μM. Animal experiments have shown that LHP has a strong hypotensive effect on rats with essential hypertension, but the hypotensive effect gradually weakens within 6-8 hours, and the blood pressure of rats basically returns to the original level after 24 hours.
[0019] (2) LHP has two amide bonds, and the ketomethyl group (-COCH 2 -) group substitution ( figure 1 ), the formed ketopeptide LHP-X was organically synthesized.
[0020] (3) The improved peptidomimetic LHP-X was in the range of pH 5-9. As the pH increased, the solubility of LHP-X decreased, and the solubility of LHP-X was higher than that of LHP. Its lipid The solubility coefficient is increased by 70-80%, and the blood pressure lowering effect can be prolonged by more than 10-15.
Embodiment 2
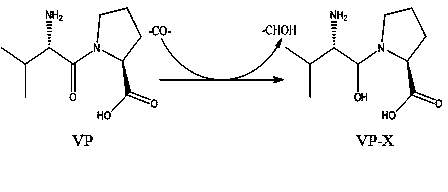
[0022] (1) Val-Pro (code-named VP) is an ACE inhibitory peptide, and its inhibitory activity against ACE in vitro is IC 50 The concentration of Val-Trp was 2.4 μM, and both middle and high doses of Val-Trp had certain blood pressure-lowering effect on SHR.
[0023] (2) The -CO- in the VP peptide bond is replaced by -CHOH- to become the peptoid product VP-X ( figure 2 ), for organic synthesis.
[0024] (3) The solubility of the improved peptidomimetic VP-X at pH 7.0 is higher than that of LHP, the fat solubility coefficient is increased by 50-60%, and the blood pressure lowering effect can be prolonged for more than 10 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com